steam at 100ºC burn worse
Moderators: Chem_Mod, Chem_Admin
-
Cynthia Ulloa
- Posts: 52
- Joined: Fri Feb 23, 2018 3:02 am
-
Ethan Breaux 2F
- Posts: 63
- Joined: Sat Sep 29, 2018 12:16 am
Re: steam at 100ºC burn worse
because it holds more energy since it has the heat energy of boiling water + the heat energy required for the phase change
-
CHEM 14B Lover
- Posts: 33
- Joined: Fri Sep 28, 2018 12:19 am
Re: steam at 100ºC burn worse
This also assumes that the number of water and steam particles are equal. Don't intuit that steam burns less because typically when you put your hadn in 100C water your hand is in contact with a lot more molecules than if you had your hand above the steam from boiling water.
-
Eva Zhao 4I
- Posts: 101
- Joined: Sun Sep 29, 2019 12:16 am
Re: steam at 100ºC burn worse
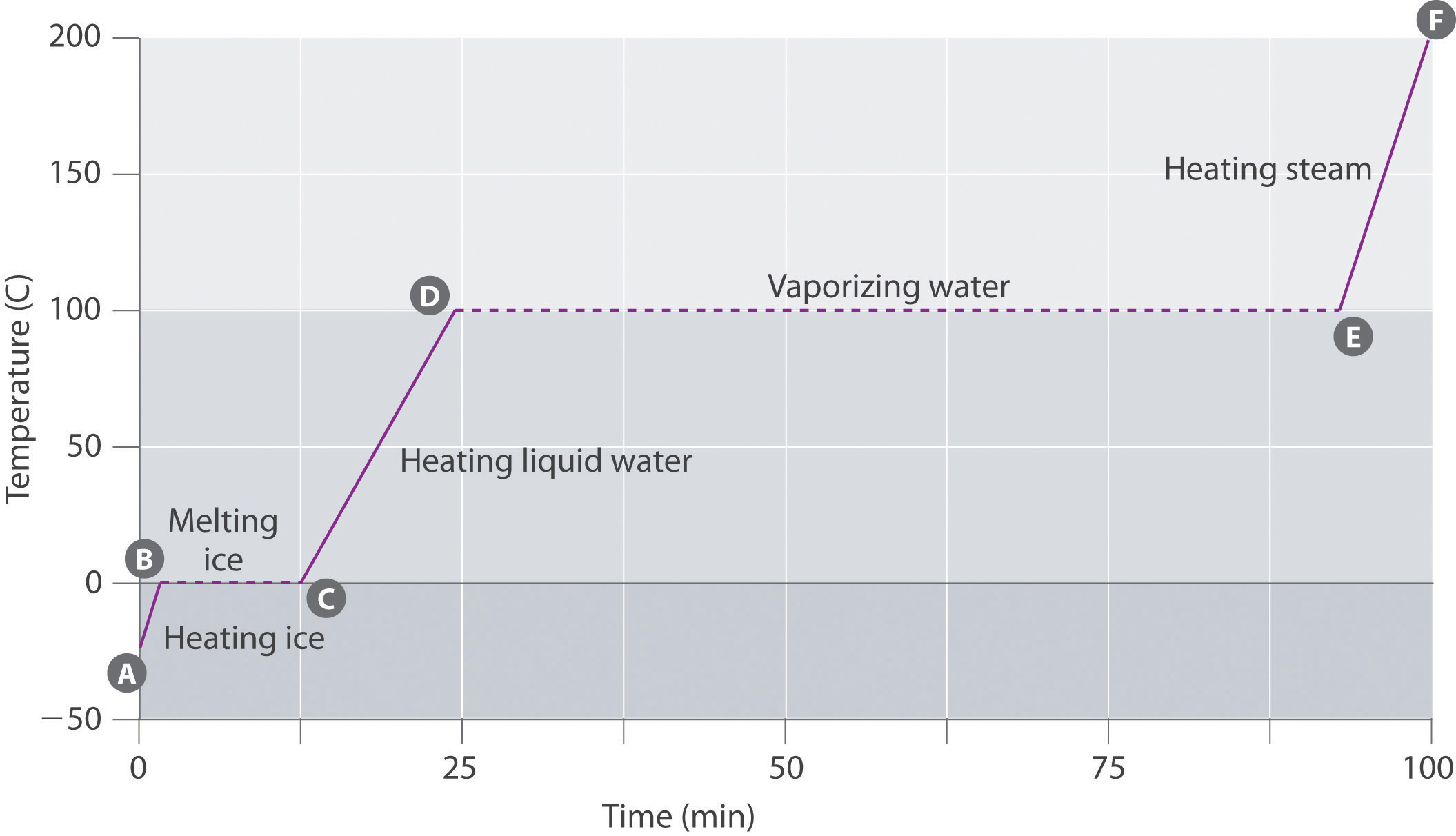
To help clarify with the help of the water heating curve, you can see in the diagram that it takes much more energy for liquid water to transition to steam than for ice to transition to liquid water. Thus, steam at 100ºC holds more energy than liquid water at 100ºC, causing worse burns.

-
Chantel_2I
- Posts: 109
- Joined: Sat Sep 07, 2019 12:19 am
Re: steam at 100ºC burn worse
When the vapor touches your hand, it undergoes a phase change to become liquid on your hand, therefore releasing even more energy in the form of heat. However, when boiling water touches your hand, it does not have that extra energy to release because it is already in the liquid phase.
-
Areli C 1L
- Posts: 95
- Joined: Wed Nov 14, 2018 12:19 am
Re: steam at 100ºC burn worse
As Ally Huang answered in another similar question, "At 100 degrees celsius, steam will produce a worse burn than boiling water because steam contains more heat energy than boiling water. It holds more energy than boiling water because it has the heat energy of boiling water and the heat energy that was required for the phase change from liquid to vapor."
So basically, the Water vapor holds more heat energy than the boiling water because in order to transition from a liquid to vapor,it requires much more energy than transitioning a solid to a liquid. (Specifically in this case water). I hope that helps.
So basically, the Water vapor holds more heat energy than the boiling water because in order to transition from a liquid to vapor,it requires much more energy than transitioning a solid to a liquid. (Specifically in this case water). I hope that helps.
-
Elizabeth Harty 1A
- Posts: 125
- Joined: Sat Jul 20, 2019 12:16 am
Re: steam at 100ºC burn worse
it has more energy bc it has the energy of boiling water and the energy needs to transition from vapor to liquid (as it touches you)
-
Minh Ngo 4G
- Posts: 137
- Joined: Thu Jul 25, 2019 12:17 am
Re: steam at 100ºC burn worse
Because the energy it takes for liquid to become steam require more energy. So when the steam touch your skin, the steam has to condensate, meaning turns back into liquid phase and when it does that, it releases all the energy
Return to “Phase Changes & Related Calculations”
Who is online
Users browsing this forum: No registered users and 7 guests

