boiling points
Moderators: Chem_Mod, Chem_Admin
-
Clarissa Nava 3H
- Posts: 60
- Joined: Fri Sep 29, 2017 7:05 am
boiling points
when reaching a boiling point in a heating curve does that mean it is already getting vaporized?
-
Michael Torres 4I
- Posts: 92
- Joined: Thu May 10, 2018 3:00 am
- Been upvoted: 1 time
Re: boiling points
To get vaporized, a substance must undergo some enthalpy of vaporization at the boiling point. Therefore, simply being at the boiling point is not enough to vaporize a substance.
-
Eva Zhao 4I
- Posts: 101
- Joined: Sun Sep 29, 2019 12:16 am
Re: boiling points
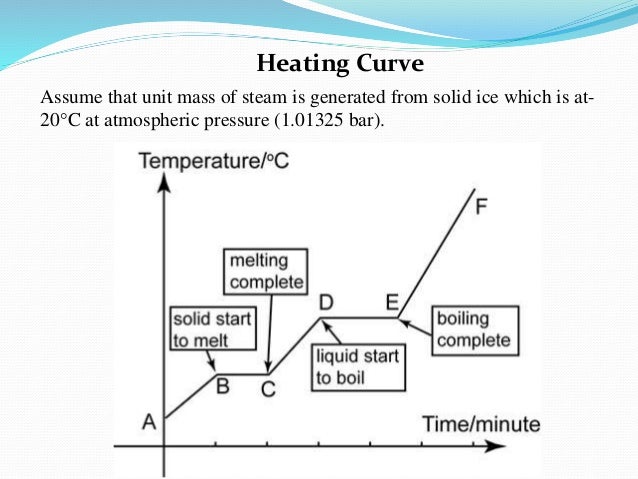
I found this diagram online which will hopefully help clarify things. You can see that it takes some time before the liquid is fully vaporized.

-
Brianna Becerra 1B
- Posts: 117
- Joined: Fri Aug 02, 2019 12:16 am
Re: boiling points
You can see from the graph that there is a moment where the water begins to boil and the temperature stays the same while heat is still being supplied. This is the phase change occurring but the vaporization is not seen until the temperature on the graph begins to rise.
-
Chantel_2I
- Posts: 109
- Joined: Sat Sep 07, 2019 12:19 am
Re: boiling points
It begins to vaporize when the temperature reaches the boiling point. It stays at that temperature until it's completely vaporized.
Return to “Phase Changes & Related Calculations”
Who is online
Users browsing this forum: No registered users and 8 guests

