Could someone explain why steam is so hot and carries more heat than boiled water? I know it might have something to do with the specific heat of vaporization.
Thank you!
Steam?
Moderators: Chem_Mod, Chem_Admin
Re: Steam?
When heating up a material, the energy can go into two places, increasing the temperature (specific heat), or changing the phase (heat of vaporization). Steam may not be more hot compared to boiling water if they are both 100ºC, but it will be carrying more energy because of the energy input in order to change the phase. This energy can be released without changing the temperature of the steam, but it will turn into water.
-
Justin Seok 2A
- Posts: 104
- Joined: Sat Aug 24, 2019 12:15 am
Re: Steam?
Essentially steam carries more energy as it takes a lot of energy to transition from liquid to vapor. So while they may have the same temperature, steam will very likely have a higher heat content due to the heat of vaporization as you mention.
-
Andrew Liang 1I
- Posts: 105
- Joined: Fri Aug 30, 2019 12:18 am
Re: Steam?
Steam has a lot of energy compared to liquid water because of its high vaporization energy. Liquid water needs to obtain a lot of energy in order for it to break the bonds and change phases from liquid to gas
-
Eva Zhao 4I
- Posts: 101
- Joined: Sun Sep 29, 2019 12:16 am
Re: Steam?
For a more visual representation through the heating curve of water, you can see that transitioning from liquid to vapor requires a lot more energy than it does from solid to liquid. Thus, steam carries more heat than boiled water.
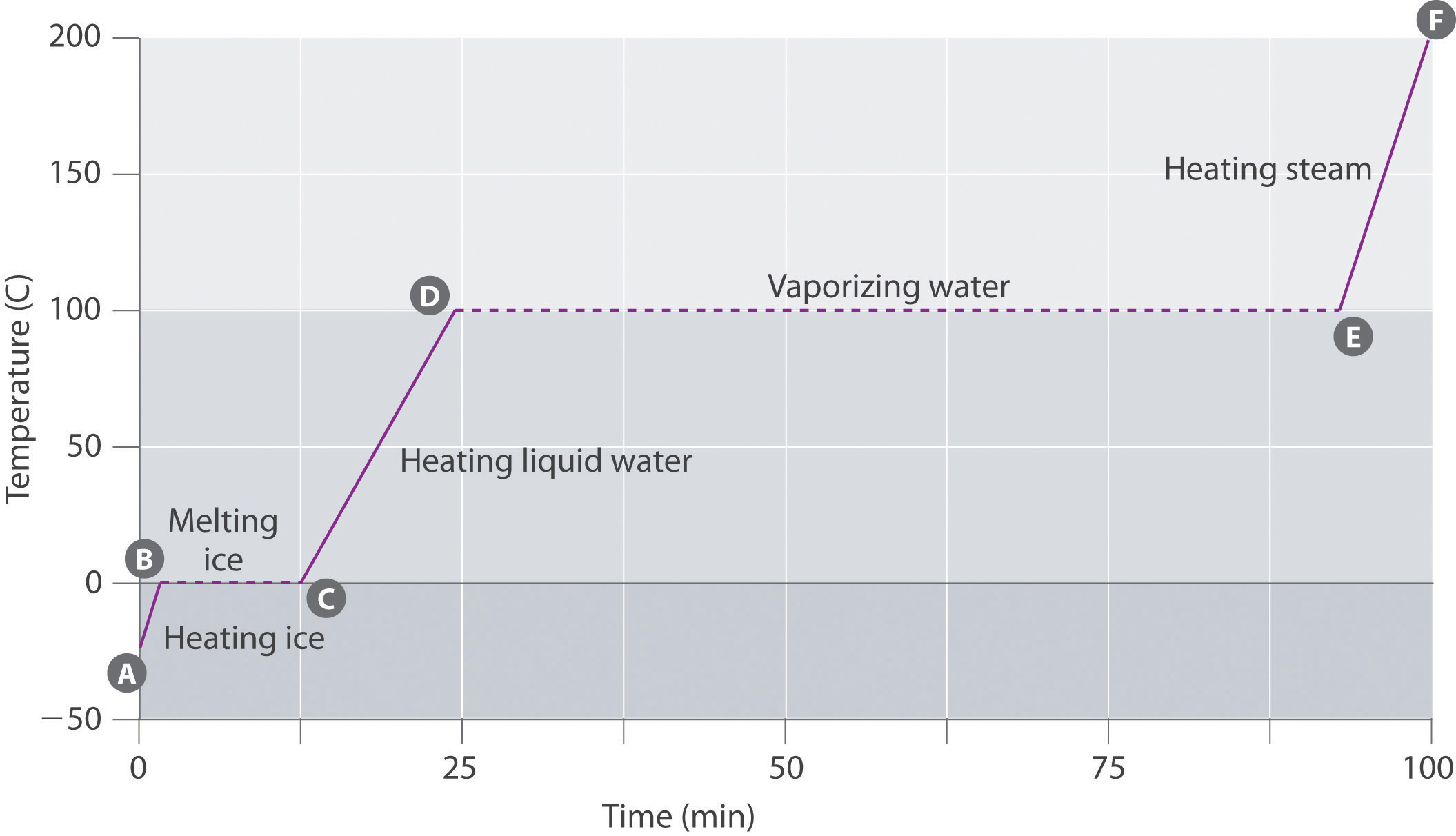

-
Mitchell Koss 4G
- Posts: 128
- Joined: Sat Jul 20, 2019 12:17 am
-
Jainam Shah 4I
- Posts: 130
- Joined: Fri Aug 30, 2019 12:16 am
Re: Steam?
The energy needed to from boiling water to steam is quite high. While steam and boiling water may not have a considerable difference in temperature when the steam cools down it will release a lot of energy as it condenses, because when the steam was formed it needed a lot of energy. When that steam condenses onto your skin it burns more severely, because its releasing a lot of energy.
Re: Steam?
will steam be on the midterm? and if so what should we know about it??? All I know is man that stuff burns lol
-
Chris Charton 1B
- Posts: 69
- Joined: Mon Jun 17, 2019 7:23 am
Re: Steam?
In order to break the hydrogen bonds found in liquid water, quite a bit of additional energy is required to be added to change water's phase from liquid to gas. This energy stays with the vaporized water molecules and it is released when it condenses back to liquid.
Return to “Phase Changes & Related Calculations”
Who is online
Users browsing this forum: No registered users and 8 guests

