melting
Moderators: Chem_Mod, Chem_Admin
-
jonathan chi 1J
- Posts: 105
- Joined: Fri Sep 24, 2021 6:02 am
-
Alan Nguyen 2I
- Posts: 100
- Joined: Fri Sep 24, 2021 6:43 am
Re: melting
Melting would me an endothermic process. Heat energy would be absorbed during melting to break intermolecular forces between the water molecules. The reaction needs to take in heat to proceed, making it endothermic. Hope this helps!
-
Achyutha Kodavatikanti_3H
- Posts: 51
- Joined: Mon Jan 03, 2022 9:33 pm
Re: melting
Melting is endothermic as breaking hydrogen bonds requires energy. solid --> liquid means hydrogen bonds are broken
Re: melting
Melting is an endothermic process because heat must be added to the system in order for the phase change to occur. This also implies that freezing and condensation are exothermic processes. Hope this helps!
-
Amy Wong 2F
- Posts: 50
- Joined: Mon Jan 03, 2022 8:48 pm
Re: melting
Melting is endothermic because energy is required to break the bonds of a solid to change into a liquid.
Also, if you think about an ice cube melting, you need to apply heat to melt the ice cube. If you take away heat, the ice stays frozen.
Also, if you think about an ice cube melting, you need to apply heat to melt the ice cube. If you take away heat, the ice stays frozen.
-
Neha Mukund
- Posts: 109
- Joined: Fri Sep 24, 2021 5:23 am
Re: melting
Melting is endothermic because heat is drawn in from the surroundings (in order to melt, an input of energy is needed to break the bonds).
-
Gianna Greco 1G
- Posts: 101
- Joined: Fri Sep 24, 2021 6:54 am
Re: melting
Hi! Melting is endothermic because energy in the form of heat is required in order for it to occur.
-
Elaine Steinberg 3H
- Posts: 105
- Joined: Fri Sep 24, 2021 6:47 am
Re: melting
Hi! Though you're not completely breaking intermolecular bonds (like the transition from liquid-->gas would), they are being weakened so that the molecules can move around more freely. This requires an input of energy so therefore it would be an endothermic process.
-
Milan Vognarek 1A
- Posts: 21
- Joined: Mon Jan 03, 2022 10:29 am
Re: melting
Melting is an endothermic process since heat is absorbed in order to break the bonds holding the atoms or molecules together.
-
Aaron Kim 1J
- Posts: 106
- Joined: Fri Sep 24, 2021 5:27 am
Re: melting
Melting is an endothermic process since heat is absorbed in the process. This heat energy is used in breaking bonds and is responsible for the phase change.
-
Ashley Hiti 1K
- Posts: 139
- Joined: Fri Sep 24, 2021 5:10 am
Re: melting
Melting is endothermic because heat is absorbed. One mnemonic that helps me is EN sounds like IN so heat goes INTO the system IN ENdothermic reactions and EXO sounds like EXIT so heat EXITS the system in EXOthermic reactions.
-
Terrence Chi
- Posts: 99
- Joined: Fri Sep 24, 2021 6:06 am
Re: melting
Hi, melting is endothermic because the ice consumes energy (heat), which allows a transition to occur. The energy is released when the bonds form to make carbon dioxide and water. Hope this helps!
-
Sidharth Paparaju 3B
- Posts: 167
- Joined: Fri Sep 24, 2021 5:14 am
Re: melting
Melting is endothermic since its converting a solid to a liquid phase, or a low energy to high energy state (the average kinetic energy of molecules is higher in liquid state versus solid).
-
Diana Avalos
- Posts: 55
- Joined: Tue Feb 16, 2021 12:15 am
Re: melting
Melting(also known as fusion) is an endothermic phase change as energy(heat) is required for a solid to become a liquid!
Re: melting
melting would be considered an endothermic reaction because heat is required for a solid to melt into a liquid.
Re: melting
Melting is an endothermic process because energy is required to heat up a substance to its melting point, then more energy is required to break the intermolecular forces of the solid.
-
Daniel Tabibian 3K
- Posts: 107
- Joined: Fri Sep 24, 2021 5:02 am
Re: melting
Melting is endothermic because it takes in energy in order to break down hydrogen bonds.
-
Amy Huynh 1B
- Posts: 103
- Joined: Fri Sep 24, 2021 6:58 am
Re: melting
Melting would be endothermic because heat needs to be added so that the bonds can break and enable the compound to undergo melting.
-
Ivy Vo Dis 1C
- Posts: 100
- Joined: Fri Sep 24, 2021 5:48 am
Re: melting
Melting is an endothermic process. This is because the ice will absorb heat energy in order to break the hydrogen bonds in the ice. By doing so, the solid ice will turn into a liquid water.
-
Joellen 1B
- Posts: 104
- Joined: Fri Sep 24, 2021 6:24 am
Re: melting
Melting is endothermic because it absorbs heat in order to change from a solid to a liquid. Take ice for example. It goes from solid to liquid when it absorbs heat.
-
Chelsea Tran 3H
- Posts: 50
- Joined: Mon Feb 22, 2021 12:15 am
Re: melting
Melting is an endothermic reaction since it requires an input of heat energy from surroundings to break the intermolecular forces holding the molecules together as a solid.
-
Brandon Yu
- Posts: 121
- Joined: Fri Sep 24, 2021 6:07 am
-
Sophie Cresitello 1B
- Posts: 50
- Joined: Mon Jan 03, 2022 10:31 am
Re: melting
Melting is an endothermic process. Going from a solid to a liquid requires breaking hydrogen bonds, and breaking hydrogen bonds requires energy. So during melting, energy must be absorbed and there for the process is endothermic.
-
Bela Patel 2B
- Posts: 85
- Joined: Fri Sep 24, 2021 6:40 am
Re: melting
Melting is endothermic because heat is absorbed during the melting process to break apart the forces between the hydrogen bonds. Since it requires heat and absorbs it, it is constituted as an endothermic reaction.
-
taline krumian 1L
- Posts: 50
- Joined: Mon Jan 03, 2022 10:58 am
Re: melting
When a substance melts, it absorbs energy from its surroundings. Therefore, the reaction is endothermic.
-
Jelix Tsan 2H
- Posts: 109
- Joined: Fri Sep 24, 2021 7:25 am
-
Samantha Quevedo 2L
- Posts: 100
- Joined: Fri Sep 24, 2021 6:00 am
-
Ashrita Singh 2F
- Posts: 111
- Joined: Fri Sep 24, 2021 6:33 am
-
Sabira Mohammed 3I
- Posts: 100
- Joined: Fri Sep 24, 2021 5:34 am
Re: melting
Melting is endothermic because energy/heat needs to be absorbed in order to break the intermolecular forces between molecules
Re: melting
Melting would be an endothermic reaction because it requires a continuous input of heat to change phases.
Hope this helps!
Hope this helps!
-
Isabelle Kim 3E
- Posts: 131
- Joined: Fri Sep 24, 2021 6:17 am
- Been upvoted: 1 time
Re: melting
Melting is an endothermic reaction because it requires heat from its direct surroundings in the process of breaking down the chemical bonds that made up the initial solid. Hence, the overall change in enthalpy is a positive one.
-
Martha Avila 1I
- Posts: 100
- Joined: Fri Sep 24, 2021 6:21 am
- Been upvoted: 1 time
Re: melting
Hello! Melting is an endothermic process because in order for the phase change to occur heat is required. This heat will break down the solid turning it into a liquid. Hope this helps!
-
Caitlyn Lo 2F
- Posts: 99
- Joined: Fri Sep 24, 2021 6:06 am
-
Rebecca Preusch 2C
- Posts: 101
- Joined: Fri Sep 24, 2021 6:26 am
-
SerenaSabedra
- Posts: 102
- Joined: Fri Sep 24, 2021 7:05 am
Re: melting
Solids melting to liquids is an endothermic process. In order to break the bonds in a solid substance, energy in the form of heat must be absorbed. When heat is absorbed in a reaction, it is endothermic.
-
Sasha Gladkikh 2A
- Posts: 190
- Joined: Fri Sep 24, 2021 5:50 am
- Been upvoted: 3 times
Re: melting
Hi,
I have attached the phase change diagram as reference:
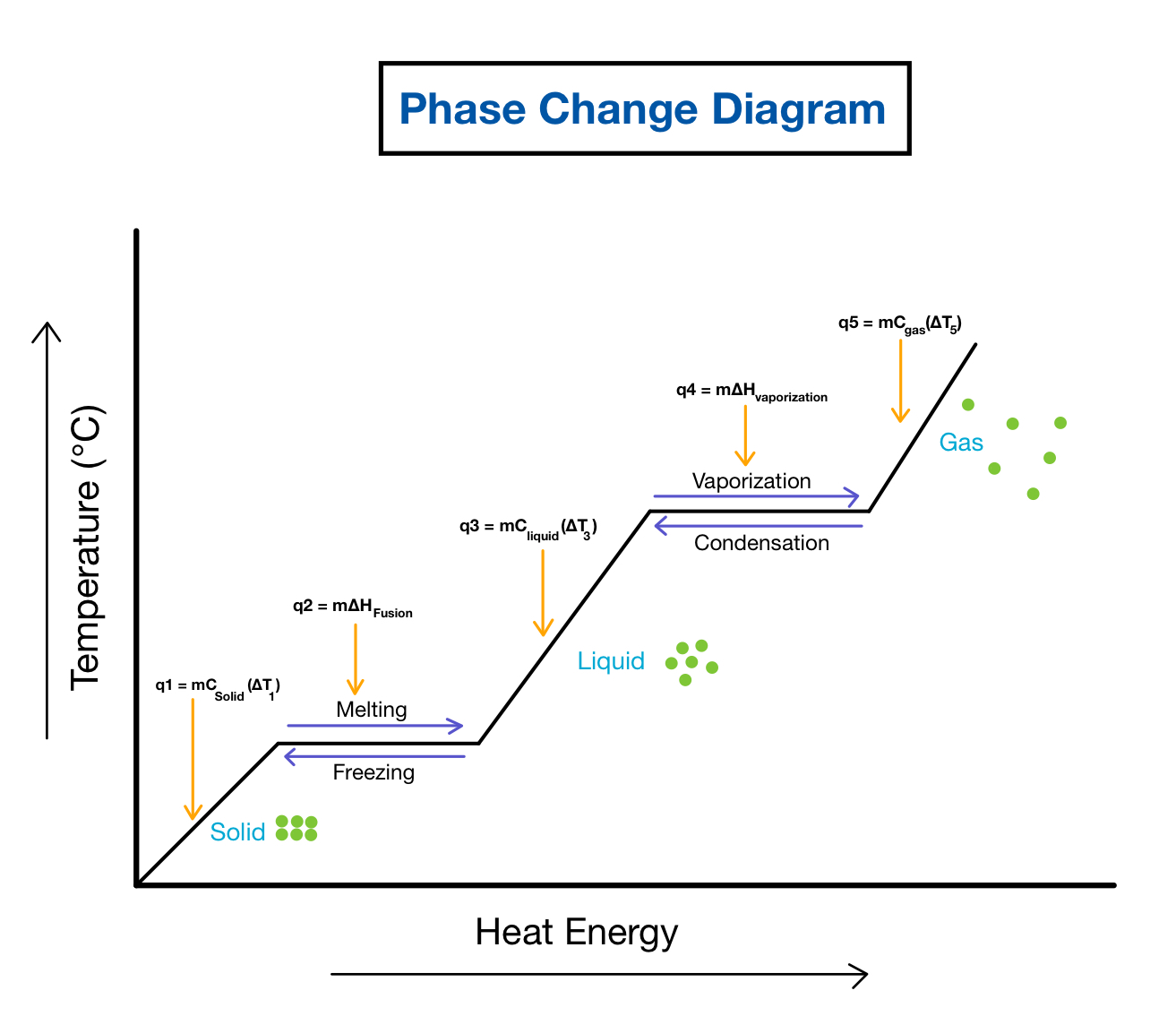
In order to determine if a phase change is endothermic or exothermic, I simply follow this trick:
• If the phase change occurs from left to right along the diagram (e.g., from solid to liquid), the phase change is endothermic.
• Conversely, if the phase change occurs from right to left along the diagram (e.g., liquid to solid), the phase change is exothermic.
*The x-axis represents heat added into the system; so, going toward the right of the diagram indicates an addition of heat (endothermic) and going toward the left of the diagram indicates a release of heat (exothermic).
Hope this helps!
I have attached the phase change diagram as reference:

In order to determine if a phase change is endothermic or exothermic, I simply follow this trick:
• If the phase change occurs from left to right along the diagram (e.g., from solid to liquid), the phase change is endothermic.
• Conversely, if the phase change occurs from right to left along the diagram (e.g., liquid to solid), the phase change is exothermic.
*The x-axis represents heat added into the system; so, going toward the right of the diagram indicates an addition of heat (endothermic) and going toward the left of the diagram indicates a release of heat (exothermic).
Hope this helps!
-
Talia Tam 3L
- Posts: 107
- Joined: Fri Sep 24, 2021 5:38 am
-
JasmineReyes-2K
- Posts: 97
- Joined: Fri Sep 24, 2021 6:12 am
-
Aryan Gajjar 3D
- Posts: 114
- Joined: Fri Sep 24, 2021 6:05 am
- Been upvoted: 1 time
Re: melting
The process is endothermic since the material is melting, hence the energy change will be positive. Energy is used only to change the phase of a substance during melting; it is not used to change the temperature of a substance.
Re: melting
Melting would be an endothermic process since heat needs to be added into the system in order for bonds to be broken.
-
RCortez_1A
- Posts: 26
- Joined: Wed Feb 17, 2021 12:20 am
-
Yewon Jang 3K
- Posts: 101
- Joined: Fri Sep 24, 2021 5:36 am
Re: melting
Melting is an endothermic process because it requires energy for this process to occur. Without heat added to the reaction, nothing would happen. When you want to melt solids into liquids you have to add heat because it wouldn't just melt on its own.
-
Isha Tripathi 2F
- Posts: 51
- Joined: Wed Feb 17, 2021 12:15 am
-
Genelle Marcelino-Searles 2G
- Posts: 102
- Joined: Sun Sep 26, 2021 5:00 am
-
Brenda Tran 3C
- Posts: 109
- Joined: Fri Sep 24, 2021 6:32 am
Re: melting
Melting is an endothermic because not only are bonds being broken, but melting required absorption of heat. For example, an ice cube melting occurs because heat is being absorbed. This means that energy is being taken into the system, so it is endothermic.
-
Desiree Eshraghi 3A
- Posts: 136
- Joined: Fri Sep 24, 2021 6:12 am
Re: melting
Melting is endothermic since it requires energy. Think about - > ice melts when it gets warmer. Therefore, change H is positive. Hope this helps.
-
Michael 1G
- Posts: 101
- Joined: Fri Sep 24, 2021 6:16 am
- Been upvoted: 1 time
Re: melting
Since ice is experiencing a phase change from solid to liquid, heat is required to be absorbed by the ice. Anytime heat is absorbed, the process is endothermic.
-
Mia Orr 3B
- Posts: 103
- Joined: Fri Sep 24, 2021 7:11 am
Re: melting
Melting is endothermic because energy is required in order for it to occur. Hope this helps!
-
Lauren Wasef 3C
- Posts: 102
- Joined: Fri Sep 24, 2021 5:29 am
Re: melting
Since heat is required to melt something, then it is endothermic! The solid takes up the heat in order for it to melt and therefore makes the surroundings cool.
-
Polo Morales 3C
- Posts: 103
- Joined: Fri Sep 24, 2021 7:01 am
Re: melting
Melting is an endothermic process. Remember that energy is required to break the intermolecular attractions of liquid molecules. Therefore, the liquid is taking up energy from its environment as it becomes a gas. Hope this helps!
-
Polo Morales 3C
- Posts: 103
- Joined: Fri Sep 24, 2021 7:01 am
Re: melting
Melting is an endothermic process. Remember that energy is required to break the intermolecular attractions of liquid molecules. Therefore, the liquid is taking up energy from its environment as it becomes a gas. Hope this helps!
-
dahlia Faruque
- Posts: 77
- Joined: Fri Sep 24, 2021 6:37 am
-
Jonathan Shyu 3L
- Posts: 102
- Joined: Fri Sep 24, 2021 5:07 am
Re: melting
Melting is endothermic because it takes heat to melt. With water, going from a solid (ice) to a liquid (normal water) requires heat, thus a positive delta h and an endothermic reaction.
-
Abu Zhang 2D
- Posts: 37
- Joined: Mon Jan 03, 2022 8:42 pm
Re: melting
As far as I know, melting is an endothermic process as phase change from solid to liquid requires energy to proceed. While it's melting, the temperature is not changing. Once melting is complete, the temperature will continue to increase.
-
Samantha Toscano 2C
- Posts: 106
- Joined: Wed Feb 17, 2021 12:24 am
-
ajguerrero
- Posts: 24
- Joined: Fri Sep 24, 2021 6:28 am
-
Cynthia_L_2C
- Posts: 99
- Joined: Fri Sep 24, 2021 7:25 am
Re: melting
From my understanding, melting is an endothermic process because it requires heat for the phase changes.
-
Daniela G 2C
- Posts: 92
- Joined: Fri Sep 24, 2021 5:06 am
-
Nyah Zhang 1E
- Posts: 51
- Joined: Mon Jan 03, 2022 10:42 am
Re: melting
Melting is an endothermic reaction. Heat needs to be absorbed in order for have a phase change from solid to liquid.
-
Warren Jolicoeur 1B
- Posts: 100
- Joined: Fri Sep 24, 2021 5:37 am
-
rachelsjordan 1K
- Posts: 106
- Joined: Fri Sep 24, 2021 6:35 am
-
Amy Jordan 2A
- Posts: 104
- Joined: Fri Sep 24, 2021 6:23 am
-
Jordyn Lee 1J
- Posts: 106
- Joined: Fri Sep 24, 2021 7:08 am
-
Anjali Botcha 3B
- Posts: 50
- Joined: Mon Jan 03, 2022 9:18 pm
Re: melting
Melting is endothermic because energy is added to break bonds to go from a solid to a liquid.
-
Nicola Higgins 14B
- Posts: 103
- Joined: Fri Sep 24, 2021 5:41 am
Re: melting
Melting is endothermic. To decide whether something is exothermic or endothermic, think "does it need heat to occur?" In this case, we know that a solid cannot melt without energy in the form of heat. On the contrary, a process such as freezing does NOT need heat to occur and is therefore exothermic.
-
Nathan Morgan 2C
- Posts: 104
- Joined: Fri Sep 24, 2021 7:16 am
Re: melting
Melting would be an endothermic reaction because when something melts it is absorbing energy which is what allows for a phase change to happen.
-
andreagutierrez 3K
- Posts: 49
- Joined: Mon Jan 03, 2022 9:39 pm
Re: melting
Melting is endothermic as heat must be added to the system in order for it to actually melt.
-
Justin An 2L
- Posts: 102
- Joined: Fri Sep 24, 2021 6:14 am
Re: melting
Melting is an endothermic reaction because heat is being absorbed, therefore the change in enthalpy is positive
-
Guadalupe_3B
- Posts: 51
- Joined: Wed Nov 25, 2020 12:19 am
Re: melting
Melting is an endothermic reaction because the ice absorbs heat/energy, which causes a change to occur.
-
Guadalupe_3B
- Posts: 51
- Joined: Wed Nov 25, 2020 12:19 am
Re: melting
Melting is an endothermic reaction because the ice absorbs heat/energy, which causes a change to occur.
-
Jack Van Ryan 1A
- Posts: 103
- Joined: Fri Sep 24, 2021 5:23 am
Re: melting
jonathan chi 1J wrote:Is melting endothermic or exothermic?
it requires heat, so endothermic.
-
Ellen Brock 2I
- Posts: 100
- Joined: Fri Sep 24, 2021 5:34 am
Re: melting
Endothermic! The solid is breaking down bonds so that it can become a liquid which means it needs to absorb heat, hence endothermic!
-
Kristen Bansil 1G
- Posts: 105
- Joined: Fri Sep 24, 2021 7:18 am
Re: melting
Melting is an endothermic process because it is breaking down bonds which requires it to absorb heat :)
-
Kristen Bansil 1G
- Posts: 105
- Joined: Fri Sep 24, 2021 7:18 am
Re: melting
Melting is an endothermic process because it is breaking down bonds which requires it to absorb heat :)
-
Sophia Manos 1F
- Posts: 52
- Joined: Mon Jan 03, 2022 10:45 am
Re: melting
Because melting requires the breaking of bonds and absorption of heat, it is considered endothermic.
-
Shannon Clark 1F
- Posts: 112
- Joined: Fri Sep 24, 2021 6:00 am
Re: melting
Melting is an endothermic because it requires heat! The substance being heated causes it to melt!
-
kayleec1004
- Posts: 74
- Joined: Fri Sep 24, 2021 6:57 am
Re: melting
Melting is an endothermic process. Heat energy is absorbed during melting stage in order to break intermolecular forces between the H2O molecules. The reaction needs to absorb heat as energy to proceed forward. breaking bonds = endothermic
-
Andrew_Ramirez
- Posts: 89
- Joined: Fri Sep 24, 2021 7:16 am
Re: melting
Melting is an endothermic reaction because it is caused by an increase of heat in the system as well as an increase in entropy.
-
Aeson Salcedo 3L
- Posts: 50
- Joined: Wed Nov 11, 2020 12:18 am
-
Amanda Tran 1D
- Posts: 101
- Joined: Fri Sep 24, 2021 7:10 am
- Been upvoted: 1 time
-
Kimberly_Wu_3H
- Posts: 36
- Joined: Mon Jan 09, 2023 10:09 am
-
Leily Garcia 1C
- Posts: 37
- Joined: Mon Jan 09, 2023 2:31 am
Re: melting
Melting is an endothermic process because in order for one to turn from a solid to a liquid, there has to be energy absorbed (heat) to make it change from one state to another.
-
Morganne Malloy 1l
- Posts: 41
- Joined: Mon Jan 09, 2023 8:29 am
Re: melting
I think of it as anything breaking down is endothermic and anything building back up is exothermic.
-
Hope_Hayashida_3E
- Posts: 37
- Joined: Mon Jan 09, 2023 10:00 am
Re: melting
Melting is endothermic because heat is needed in order for it to occur. Think of ice. In order for it to melt, it has to absorb heat.
-
Hien Nguyen 2G
- Posts: 34
- Joined: Mon Jan 09, 2023 9:14 am
Re: melting
melting is endothermic because it requires heat absorption in order to break down the bonds.
-
Natalie Hurd 1H
- Posts: 35
- Joined: Mon Jan 09, 2023 8:23 am
Re: melting
Melting is endothermic! It requires heat in order to break bonds in the solid state to form a liquid.
-
nikita_manyak28
- Posts: 34
- Joined: Mon Jan 09, 2023 2:49 am
Re: melting
An endothermic process is one that requires energy while an exothermic process is one that releases energy. Melting is an endothermic process because it requires energy to break the bonds that are holding the molecules together while they are solid.
-
VictoriaPietrusiew2H
- Posts: 35
- Joined: Wed Feb 09, 2022 8:28 pm
Re: melting
Melting is an endothermic process! This is because melting requires the input of energy in order to break the bonds. Exothermic process are processes that release energy when bonds form. So freezing or vaporization would be exothermic!
-
Frankie Xu 2H
- Posts: 31
- Joined: Mon Jan 09, 2023 9:18 am
Re: melting
Melting is an endothermic process as it requires energy to break bonds and the phase change is from a solid to a liquid.
-
Dominic Pham 3A
- Posts: 35
- Joined: Mon Jan 09, 2023 9:42 am
Re: melting
When thinking about endothermic v. exothermic, think of bonds breaking and forming as we've learned in relation to the equilibrium constant shifts due to temperature. When ice is melting, bonds are broken and thus require energy which results in K shifting to the right to favor the creation of products.
Return to “Phase Changes & Related Calculations”
Who is online
Users browsing this forum: No registered users and 10 guests

