delta H
Moderators: Chem_Mod, Chem_Admin
-
Rachel Martinez 1A
- Posts: 85
- Joined: Fri Sep 24, 2021 5:45 am
-
Abigail Tran 14a
- Posts: 126
- Joined: Fri Sep 24, 2021 7:12 am
Re: delta H
when delta h is negative that means it is losing heat and when delta h is positive it means gaining heat
-
Serene Liu 3H
- Posts: 102
- Joined: Fri Sep 24, 2021 5:06 am
Re: delta H
Delta H is the change in enthalpy of a system in a reaction, so when it's negative the system released heat (exothermic) versus when it's negative the system gained heat (endothermic).
-
Chiara Frank
- Posts: 100
- Joined: Fri Sep 24, 2021 7:03 am
Re: delta H
Hi! Delta H refers to the change in enthalpy or total heat content of the system. Thus, when delta H is negative, this means the system is exothermic as the system is releasing or losing heat, whereas a positive delta H represents an endothermic reaction as the reaction is gaining or absorbing heat into the system. I hope this was helpful!
-
Anubhav_Chandla1G
- Posts: 104
- Joined: Fri Sep 24, 2021 6:40 am
- Been upvoted: 1 time
Re: delta H
Only the enthalpy change (H) may be measured. Heat moves from a system to its surroundings when H is negative; heat flows into a system from its surroundings when H is positive.
-
Sophia Manos 1F
- Posts: 52
- Joined: Mon Jan 03, 2022 10:45 am
Re: delta H
Delta H directly correlates to the change in enthalpy, or the gaining or losing of heat! A negative delta H indicates that heat has exited the system, while a positive delta H indicates that that has entered the system.
-
SofiaMammaro-1K
- Posts: 107
- Joined: Fri Sep 24, 2021 7:31 am
Re: delta H
A negative ΔH means that heat flows from a system to its surroundings; a positive ΔH means that heat flows into a system from its surroundings.
-
Ellie Fox 2K
- Posts: 100
- Joined: Fri Sep 24, 2021 5:28 am
Re: delta H
Delta H is reaction enthalpy. When it is negative, that means heat is being lost and the reaction is exothermic. When it is positive, that means heat is gained and the reaction is endothermic.
-
Caitlin_Doak_2H
- Posts: 52
- Joined: Wed Feb 17, 2021 12:18 am
Re: delta H
Negative means the system is releasing heat, positive means the system is absorbing heat
-
Xzandalyn Kallstrom 2C
- Posts: 53
- Joined: Mon Jan 03, 2022 8:54 pm
Re: delta H
Delta H is the reaction enthalpy, which is negative when it is losing heat, or expelling heat to the system (exothermic) if delta H is positive, it is taking heat from the surroundings (endothermic).
-
Amy Shimizu 1J
- Posts: 100
- Joined: Fri Sep 24, 2021 6:33 am
- Been upvoted: 1 time
Re: delta H
Delta H represents the change in enthalpy, or energy in the form of heat. When delta H is negative, it means that heat energy was released or lost, and when delta H is positive, it means heat energy was absorbed or gained.
-
Rachel Bartley 2B
- Posts: 104
- Joined: Fri Sep 24, 2021 6:06 am
Re: delta H
When delta H is negative, that means heat is leaving a system. When it is positive, heat is entering the system.
-
Caitlin Gee 2K
- Posts: 101
- Joined: Fri Sep 24, 2021 6:20 am
- Been upvoted: 1 time
Re: delta H
delta H refers to the change in enthalpy of the system. If it is negative, the system is losing heat and if it is positive, the system is gaining heat.
-
JasmineReyes-2K
- Posts: 97
- Joined: Fri Sep 24, 2021 6:12 am
-
Collin Le 3I
- Posts: 52
- Joined: Mon Jan 03, 2022 9:35 pm
Re: delta H
When delta H is negative, heat is leaving the system. When delta H is positive, heat is entering the system.
-
Madison Yee 2B
- Posts: 101
- Joined: Fri Sep 24, 2021 5:33 am
Re: delta H
delta H being negative signifies that the reaction is exothermic and that heat is being released. H being positive indicates that the reaction is endothermic and heat is required for the reaction to occur.
-
Mia Orr 3B
- Posts: 103
- Joined: Fri Sep 24, 2021 7:11 am
Re: delta H
A negative ΔH value means that a reaction is exothermic and indicates that heat is leaving a system. A positive ΔH value means that a reaction is endothermic and heat is entering a system. Hope this helps!
-
Jelix Tsan 2H
- Posts: 109
- Joined: Fri Sep 24, 2021 7:25 am
Re: delta H
When delta H is negative, it means there is a net loss of heat from the system. When delta H is positive, it means there is a gain of heat.
Re: delta H
Delta H is the change in energy, so when it is negative that means the system loses heat, and when it is positive there has been a gain in heat.
-
Sean Sanders 1E
- Posts: 104
- Joined: Fri Sep 24, 2021 5:20 am
- Been upvoted: 1 time
Re: delta H
Negative delta H means that heat is lost (exothermic). Positive delta H means that heat is gained (endothermic).
-
Bela Patel 2B
- Posts: 85
- Joined: Fri Sep 24, 2021 6:40 am
Re: delta H
If it is negative, then it is losing heat but if it is positive then it is gaining heat.
Re: delta H
Delta H refers to the change in enthalpy, so when it is negative it means heat is lost and when it is positive it means heat is being gained!
-
Amy Huynh 1B
- Posts: 103
- Joined: Fri Sep 24, 2021 6:58 am
Re: delta H
delta H represents change in enthalpy. when it's negative, the reaction is exothermic or the system loses heat. when it's positive, the reaction is endothermic or the system gains heat.
-
Sofia Potter 1I
- Posts: 39
- Joined: Wed Oct 06, 2021 5:02 am
Re: delta H
When delta H is negative, the reaction is exothermic (losing heat), which also generally means it occurs spontaneously. The opposite goes for a positive delta H, where the reaction is endothermic and generally does not occur spontaneously.
-
Natalie Quilala 1I
- Posts: 105
- Joined: Fri Sep 24, 2021 5:28 am
Re: delta H
Negative delta H is exothermic (loss of heat) and positive delta H is endothermic (gaining heat in the system).
-
Shannon Clark 1F
- Posts: 112
- Joined: Fri Sep 24, 2021 6:00 am
Re: delta H
When delta H is negative it means there is a loss of heat (exothermic) , and when it’s positive is means the reaction is endothermic and is absorbing heat!
Re: delta H
Hello!
When delta H is negative it means that there is a loss of heat (exothermic) and a gain of heat (endothermic) when it is positive! The heat is gained/ lost by the system! I hope this helped!
When delta H is negative it means that there is a loss of heat (exothermic) and a gain of heat (endothermic) when it is positive! The heat is gained/ lost by the system! I hope this helped!
-
Minh Nguyen 3H
- Posts: 135
- Joined: Wed Sep 30, 2020 9:36 pm
Re: delta H
delta H refers to change in heat, meaning that a negative value correlates to a decrease in heat, while a positive value correlates to an increase in heat.
-
Andrew Yim 1K
- Posts: 52
- Joined: Mon Jan 03, 2022 11:07 am
Re: delta H
Hi,
Delta H represents a change in enthalpy in the system. If it is negative, the system is exothermic, if it is positive, the system is endothermic.
Delta H represents a change in enthalpy in the system. If it is negative, the system is exothermic, if it is positive, the system is endothermic.
-
Nithya Narapa Reddy
- Posts: 100
- Joined: Fri Sep 24, 2021 6:47 am
Re: delta H
Delta H is the change in enthalpy for the system. Negative delta H means it is exothermic while a positive delta H means its endothermic. Exothermic is a loss of heat and Endothermic is a gain of heat.
Re: delta H
Delta H refers to the change in enthalpy for the system with negative meaning if it's negative then the system is exothermic and if it positive then the system is endothermic.
-
Bobak Pourrahimi 2L
- Posts: 108
- Joined: Fri Sep 24, 2021 6:09 am
Re: delta H
If delta H is negative, that indicates that heat is leaving the particular system being described, whereas a positive delta H would indicate that heat is entering the given system. It is important to be careful as to not flip the two sinc eit can drastically alter the calculations one makes.
-
Jessica Sun 2I
- Posts: 102
- Joined: Fri Sep 24, 2021 7:18 am
Re: delta H
When delta H is negative, it means that enthalpy is decreasing and the reaction is exothermic because heat is being released. When delta H is positive, it means that enthalpy is increasing and the reaction is exothermic because heat is being absorbed.
-
Morgan Micallef 1A
- Posts: 104
- Joined: Fri Sep 24, 2021 5:03 am
Re: delta H
Delta H is referring to the change of heat in a system. A negative delta H means the system is losing heat, and a positive delta H means that the system is gaining heat
-
Hannah Choi 1K
- Posts: 99
- Joined: Fri Sep 24, 2021 6:07 am
Re: delta H
delta indicates change and H indicates enthalpy. Therefore, delta Hrepresents a change in enthalpy. So if delta H is negative, heat is being lost from the system (exothermic), and if delta H is positive, heat is being brought into the system (endothermic)
-
Madison Rhynhart 3H
- Posts: 99
- Joined: Fri Sep 24, 2021 6:20 am
Re: delta H
When delta H is negative the system is losing heat and when it is positive the system is gaining heat.
-
Jennifer Fuentes 2K
- Posts: 99
- Joined: Fri Sep 24, 2021 6:17 am
Re: delta H
If ∆H is negative, this means that the reaction gives off heat from reactants to products. This is favorable. If ∆S is positive, this means that the disorder of the universe is increasing from reactants to products
-
Clarence Clavite 2K
- Posts: 100
- Joined: Fri Sep 24, 2021 5:33 am
Re: delta H
When deltaH is positive that means that the system gains heat. When deltaH is negative it means that the system is losing heat.
-
Sarah Wang 1I
- Posts: 106
- Joined: Fri Sep 24, 2021 7:08 am
Re: delta H
Delta H represents the enthalpy of a reaction, which basically refers to heat. When the delta H is negative, the system released heat (think of it as subtracting heat, which is why it's negative), and when the delta H is positive, the system absorbed heat (adding heat).
-
Charlie Gravereaux
- Posts: 101
- Joined: Fri Sep 24, 2021 5:51 am
Re: delta H
delta H represents enthalpy, which represents heat of a system. For a negative delta H, we know the system is losing heat, while a positive delta H represents a gain in heat.
-
Amanda Jacobs 2B
- Posts: 49
- Joined: Mon Jan 03, 2022 8:35 pm
Re: delta H
Delta H is representative of the energy of a system. So, if delta H is positive then the system gained heat energy. If delta H is negitve then the system lost heat energy.
-
Polo Morales 3C
- Posts: 103
- Joined: Fri Sep 24, 2021 7:01 am
Re: delta H
When deltaH is negative, it means that the change in energy of the system is negative, meaning it lost energy. By that same logic, if deltaH is positive, the system has gained energy. Hope this helps!
-
Chris Korban 1D
- Posts: 107
- Joined: Fri Sep 24, 2021 6:53 am
Re: delta H
when H is negative it means system loses heat and is exothermic and if positive it is endothermic and gains heat
-
Amy Jordan 2A
- Posts: 104
- Joined: Fri Sep 24, 2021 6:23 am
Re: delta H
Hi, delta H represents change in enthalpy. When delta H is positive it's an endothermic reaction and when delta H is negative it's an exothermic reaction.
-
dericasu3a
- Posts: 49
- Joined: Fri Sep 24, 2021 6:36 am
Re: delta H
When delta H is negative, it's losing heat so it's exothermic. When delta H is positive, the system is gaining heat so it's endothermic. Delta H represents change in enthalpy.
-
Nick Oscarson 1K
- Posts: 105
- Joined: Fri Sep 24, 2021 6:28 am
Re: delta H
Delta H represents the change in enthalpy (heat). Therefore, when this value is negative the system loses heat, and when the value is positive the system gains heat.
-
Omeed Kalan
- Posts: 112
- Joined: Fri Sep 24, 2021 6:10 am
-
DArcy Perlman 1C
- Posts: 118
- Joined: Fri Sep 24, 2021 5:24 am
Re: delta H
Delta H represents the change in enthalpy or the heat content of a system, so when delta H is negative the system has lost heat (total heat decreased, represents exothermic rxn) and when it is positive the system has gained heat (total heat increased, represents endothermic rxn).
-
Kelly McFarlane
- Posts: 101
- Joined: Fri Sep 24, 2021 6:02 am
Re: delta H
Delta H is the change in enthalpy. When delta H is negative, the reaction is exothermic and when it is positive, the reaction is endothermic.
-
RobinFong_2B
- Posts: 53
- Joined: Mon Jan 03, 2022 8:44 pm
Re: delta H
negative delta H means the system has lost heat to its surroundings. Positive delta H means that the system has gained heat from its surrounding.
-
Aashna Bhandari 1L
- Posts: 102
- Joined: Fri Sep 24, 2021 7:06 am
Re: delta H
Delta H refers to the exchanges in the enthalpy. A negative delta H means the reaction is exothermic, so energy is released into the surroundings. A positive delta H means the reaction is endothermic, and energy is absorbed from the surroundings.
-
Anish_Marripati_2F
- Posts: 104
- Joined: Fri Sep 24, 2021 7:31 am
Re: delta H
When delta H is negative, the reaction is exothermic and heat is released into the surroundings. When delta H is positive, heat is going into the system and the reaction is endothermic.
Re: delta H
When delta H is negative, the reaction is exothermic. When delta H is positive, the reaction is endothermic.
-
Esther Kim
- Posts: 99
- Joined: Fri Sep 24, 2021 6:07 am
-
Benicio Rivera 1F
- Posts: 138
- Joined: Fri Sep 24, 2021 6:42 am
Re: delta H
If ∆H is negative, this means that the reaction gives off heat from reactants to products. This is favorable. When ∆H is positive it means it gains heat.
-
davis sandberg 2H
- Posts: 104
- Joined: Fri Sep 24, 2021 7:30 am
Re: delta H
-delta H means the system is losing heat, so it's exothermic. +delta H means the system is gaining heat, so it's endothermic.
-
RobinFong_2B
- Posts: 53
- Joined: Mon Jan 03, 2022 8:44 pm
Re: delta H
delta H is enthalpy. While a -H value indicates that the reaction is likely exothermic we can't be sure because it is also dependent on the sign of entropy. Taking both into account we can determine the sign of free Gibbs energy which is a better indicator of spontaneity.
-
Eszter Kovacs 1A
- Posts: 100
- Joined: Fri Sep 24, 2021 5:41 am
-
Nathan Morgan 2C
- Posts: 104
- Joined: Fri Sep 24, 2021 7:16 am
Re: delta H
Since delta H is the enthalpy of the system, if the delta H is positive, then our system has absorbed heat but if it is negative, then it has lost heat!
-
Aeson Salcedo 3L
- Posts: 50
- Joined: Wed Nov 11, 2020 12:18 am
Re: delta H
When delta H is negative there is a loss of heat (exothermic) and positive there is a gain of heat (endothermic)
Re: delta H
A negative delta H signifies exothermic and release of energy while positive is endothermic and requires energy
-
Hayden Jackson
- Posts: 100
- Joined: Fri Sep 24, 2021 6:26 am
Re: delta H
When the delta H of a system is negative that means the system is giving off heat and the reaction is exothermic. If delta H is positive the system is gaining heat and the reaction is considered endothermic.
-
Guadalupe_3B
- Posts: 51
- Joined: Wed Nov 25, 2020 12:19 am
Re: delta H
Delta H when it is negative means loss of heat and delta H when it is positive means gain of heat.
Re: delta H
A negative value indicates that the system is releasing heat, whereas a positive value indicates that the system is absorbing heat.
Re: delta H
If delta H is negative this means that the reaction gives off heat from reactants to products (Exothermic) and this is favorable. If delta H is positive this means that the reaction absorbs heat (endothermic) and this is unfavorable.
-
Kainath Kamil Dis 2K
- Posts: 103
- Joined: Fri Sep 24, 2021 5:37 am
Re: delta H
Delta H represents the loss and gain of heat; negative sign means it is loosing heat and vise versa.
Re: delta H
When delta H is negative it is losing heat and when delta H is positive it is absorbing or gaining heat
-
taline krumian 1L
- Posts: 50
- Joined: Mon Jan 03, 2022 10:58 am
Re: delta H
when delta H is negative, the system is losing heat (exothermic)
when delta H is positive, the system is gaining heat (endothermic)
when delta H is positive, the system is gaining heat (endothermic)
-
Ashwin Vasudevan 3A
- Posts: 121
- Joined: Fri Sep 24, 2021 5:57 am
-
Amy Huynh 1B
- Posts: 103
- Joined: Fri Sep 24, 2021 6:58 am
Re: delta H
When it is positive, a reaction is endothermic meaning it requires the addition of heat. When it is negative, a reaction is exothermic meaning it will release heat.
Re: delta H
When delta H is negative, that indicates that the reaction releases heat as a product and is called an exothermic reaction. On the other hand, when delta H is positve, this indiciates that the reaction consumes heat as a reactant, and is called an endothermic reaction.
Re: delta H
When delta H is negative, that indicates that the reaction releases heat as a product and is called an exothermic reaction. On the other hand, when delta H is positve, this indiciates that the reaction consumes heat as a reactant, and is called an endothermic reaction.
-
Jarod Miller 2E
- Posts: 34
- Joined: Mon Jan 09, 2023 9:02 am
Re: delta H
Delta H when negative means that it is losing heat which would be exothermic while when it’s positive it means that it’s gaining heat which would be endothermic.
-
Sabrina Camua 2J
- Posts: 36
- Joined: Mon Jan 09, 2023 9:26 am
Re: delta H
A negative delta H indicates a loss of heat (exothermic reaction) while a positive delta H indicates gaining heat (endothermic reaction).
-
sherwin arian 1K
- Posts: 36
- Joined: Mon Jan 09, 2023 8:37 am
Re: delta H
delta H is a change in enthalpy. If it is positive it means heat flows in if negative it means heat flows out.
-
Kimberly_Wu_3H
- Posts: 36
- Joined: Mon Jan 09, 2023 10:09 am
Re: delta H
Negative delta H means there is a loss of heat (exothermic reaction) while a positive delta H heat is gaining (endothermic reaction).
Re: delta H
delta H = enthalpy
negative = exothermic, system loses heat
positive = endothermic, system gains heat
negative = exothermic, system loses heat
positive = endothermic, system gains heat
-
Harsha Kancharla 2E
- Posts: 35
- Joined: Mon Jan 09, 2023 9:05 am
Re: delta H
Delta H represents enthalpy which is just the measurement of total heat in a system. A negative delta h means the system is losing heat (exothermic), while a positive delta h means the system is gaining heat (endothermic).
-
VictoriaPietrusiew2H
- Posts: 35
- Joined: Wed Feb 09, 2022 8:28 pm
Re: delta H
Delta H is the change in enthalpy of a system.
+ Delta H = endothermic reaction, requires heat, products have a higher enthalpy than reactants.
- Delta H = exothermic reaction, gives off heat, reactants have a higher enthalpy than products.
+ Delta H = endothermic reaction, requires heat, products have a higher enthalpy than reactants.
- Delta H = exothermic reaction, gives off heat, reactants have a higher enthalpy than products.
-
Morganne Malloy 1l
- Posts: 41
- Joined: Mon Jan 09, 2023 8:29 am
Re: delta H
When delta h is negative, it means that it is losing heat nd opposite when it is positivie
-
Lizze Cardoso 2I
- Posts: 34
- Joined: Mon Jan 09, 2023 9:24 am
Re: delta H
A positive value for delta H means that the reaction requires heat, meaning it is an endothermic reaction. A negative value for delta H indicates heat released in a reaction, which is an exothermic reaction. Both concepts, describing the release and absorption of heat, are defined as enthalpy.
-
Marina Nimnual
- Posts: 34
- Joined: Mon Jan 09, 2023 9:27 am
Re: delta H
When delta H is positive, that means the system takes in heat from the surrounding. On the other hand, when delta H is negative, is releases heat into the surrounding.
-
Shiraz Becker 2A
- Posts: 34
- Joined: Mon Jan 09, 2023 8:46 am
Re: delta H
When delta H is positive, the reaction is endothermic and is absorbing heat. When delta H is negative, the reaction is exothermic and is releasing heat.
-
Vivek Punn 1E
- Posts: 35
- Joined: Mon Jan 09, 2023 2:39 am
Re: delta H
Perhaps this may be easier to visualize with a graph. This shows the change potential energy as a reaction proceeds. It also gives us a nice visualization for the change in enthalpy. If delta H was positive we can see how this would require an input of energy (endothermic) vs if it were negative it would release energy (exothermic). In this diagram we can see that the forward reaction would be exothermic but the reverse would be endothermic. Hope that helps!
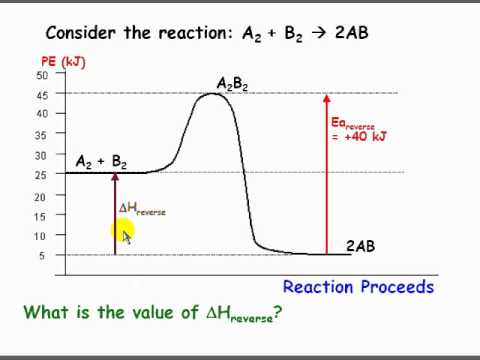

Re: delta H
Rachel Martinez 1A wrote:What does delta H mean when it is negative and what does it mean when it is positive?
A negative delta H refers to an exothermic reaction and thus release of energy. A positive delta H refers to a endothermic reaction and thus a requirement of energy.
Re: delta H
A negative delta H means the reaction is exothermic. An exothermic reaction releases heat. A positive delta H means the reaction is endothermic. An endothermic reaction uses heat.
-
parisnortega1
- Posts: 34
- Joined: Mon Jan 09, 2023 2:29 am
Re: delta H
A negative ΔH means that heat flows from a system to its surroundings; a positive ΔH means that heat flows into a system from its surroundings.
-
Jon Trujillo 3L
- Posts: 34
- Joined: Mon Jan 09, 2023 10:16 am
Re: delta H
delta H represents the change in energy during a reaction, or the difference in energy between the products and the reactants. A negative delta H means that energy is released as heat and that the reaction is exothermic while a positive delta H means that energy is absorbed to form products and that the reaction is endothermic.
-
CharlotteHilsabe 1B
- Posts: 36
- Joined: Mon Jan 09, 2023 2:24 am
Re: delta H
ΔH represents heat flow in the system. If ΔH is negative the system is exothermic and releasing energy. If ΔH is negative the system is endothermic and is absorbing and using energy.
-
Dhruvi Mehta 1L
- Posts: 34
- Joined: Mon Jan 09, 2023 8:40 am
Re: delta H
a negative delta h means that the reaction is exothermic, and energy is being released. on the other hand, a positive delta h means the reaction is endothermic, and energy is being absorbed.
-
Sydney Tanimitsu 2B
- Posts: 34
- Joined: Mon Jan 03, 2022 9:11 am
Re: delta H
A negative delta h means that there is a net loss of heat and therefore the reaction is exothermic. If the delta h is positive then the reaction would be endothermic
-
Kaylee Cheng 3E
- Posts: 36
- Joined: Mon Jan 09, 2023 10:01 am
Re: delta H
When you see a delta H, it represents that a change in enthalpy exists within a system. a negative value is loss of heat and an exothermic reaction, while a positive value is gaining heat as more energy is absorbed.
-
Kaylee Cheng 3E
- Posts: 36
- Joined: Mon Jan 09, 2023 10:01 am
Re: delta H
When you see a delta H, it represents that a change in enthalpy exists within a system. a negative value is loss of heat and an exothermic reaction, while a positive value is gaining heat as more energy is absorbed.
-
andrew_bishay_1D
- Posts: 38
- Joined: Mon Jan 09, 2023 2:34 am
Re: delta H
delta h is the change in enthalpy, or heat, for a reaction. If delta H is positive, that means there was a net gain of heat during a reaction.
-
Jennifer Jin
- Posts: 37
- Joined: Mon Jan 09, 2023 9:28 am
Re: delta H
Delta H is the total change in enthalpy. When delta H is positive it means that the reaction needs heat(endothermic), when delta H is negative it means it releases heat (exothermic).
Return to “Phase Changes & Related Calculations”
Who is online
Users browsing this forum: No registered users and 6 guests

