Spontaneity & exo/endothermic
Moderators: Chem_Mod, Chem_Admin
Re: Spontaneity & exo/endothermic
No they are not all spontaneous. This is because there could be a high activation energy required to start the reaction.
A logical example is burning wood or paper. It is an exothermic reaction because it releases heat; however, not all paper or wood spontaneously burns due to the activation energy required to start the reaction.
A logical example is burning wood or paper. It is an exothermic reaction because it releases heat; however, not all paper or wood spontaneously burns due to the activation energy required to start the reaction.
Re: Spontaneity & exo/endothermic
Not all exothermic reactions are spontaneous. A negative Delta G means that a reaction is spontaneous. The change in enthalpy can contribute to this but an exothermic reaction is not the only factor deciding if a reaction is spontaneous.
-
Averie Moore 2F
- Posts: 79
- Joined: Fri Sep 29, 2023 11:28 am
Re: Spontaneity & exo/endothermic
The Gibbs free energy of the reaction determines if it is spontaneous by calculation of a negative value.
-
Holiday Parsons 2D
- Posts: 123
- Joined: Fri Sep 29, 2023 11:07 am
Re: Spontaneity & exo/endothermic
On top of the other answers: refer to the equation 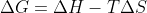 (in standard conditions). There are some values you can put into that equation that show a relationship between the variables that represent exothermic reactions that don't have to be spontaneous such as when
(in standard conditions). There are some values you can put into that equation that show a relationship between the variables that represent exothermic reactions that don't have to be spontaneous such as when  is negative and the other variables represent a non-spontaneous process (e.g, low values in entropy but with a higher temperature).
is negative and the other variables represent a non-spontaneous process (e.g, low values in entropy but with a higher temperature).
Who is online
Users browsing this forum: No registered users and 9 guests

