Find Enthalpy: 2HCl (aq) + Zn (s) --> H2(g)+ ZnCl2 (aq)
Moderators: Chem_Mod, Chem_Admin
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Find Enthalpy: 2HCl (aq) + Zn (s) --> H2(g)+ ZnCl2 (aq)
The enthalpy of the reaction is (the sum of the enthalpy of products - the sum of the enthalpy of the reactants). In this problem, 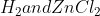 are the products. The enthalpy formation of any element (in this case is
are the products. The enthalpy formation of any element (in this case is 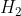 ) is 0 because
) is 0 because  \rightarrow H_{2} (g)) gives zero. Therefore, [2(-167.16)-0] - [(153.89) + 2(-167.16)] = -153.89 kJ.
gives zero. Therefore, [2(-167.16)-0] - [(153.89) + 2(-167.16)] = -153.89 kJ.
-
Victor Qiu 1C
- Posts: 106
- Joined: Wed Sep 30, 2020 9:59 pm
- Been upvoted: 2 times
Re: Find Enthalpy: 2HCl (aq) + Zn (s) --> H2(g)+ ZnCl2 (aq)
I think the equation is not correct. I think you should use
ΔHfo(Zn2+)+2ΔHfo(Cl-)-2ΔHfo(HCl)
which gives
-153.89 kJ/mol + 2 × (-167.16 kJ/mol) - 2 × (-167.16 kJ/mol)
and the result is
ΔHr = -153.89 kJ/mol
ΔHfo(Zn2+)+2ΔHfo(Cl-)-2ΔHfo(HCl)
which gives
-153.89 kJ/mol + 2 × (-167.16 kJ/mol) - 2 × (-167.16 kJ/mol)
and the result is
ΔHr = -153.89 kJ/mol
-
Daniela Santana 2L
- Posts: 103
- Joined: Wed Sep 30, 2020 9:59 pm
Re: Find Enthalpy: 2HCl (aq) + Zn (s) --> H2(g)+ ZnCl2 (aq)
In order to find the enthalpy of this reaction, you should find the sum of the products and the sum of the reactants and subtract them (sum of products - sum of reactants). You can find the respective deltaHf value in the chempendix for each compound and multiply it by their coefficient in the reaction(if they have one). I hope this helps!
Who is online
Users browsing this forum: No registered users and 6 guests

