Problem:
Use the bond enthalpies in Tables 8.6 and 8.7 to estimate the reaction enthalpy for
(b) CH3CHCH2 (g) + H2O (g) --> CH3CH(OH)CH3 (g)
I don't understand how the solutions manual determines which bonds to break and form. For this problem, 1 mol of C=C bonds and 1 mol of O-H bonds are broken and 1 mol of C-C bonds, 1 mol of C-O bonds, and 1 mol C-H bonds are formed. However, aren't there more bonds that are broken and formed than what is listed? Can someone please explain?
Problem 8.75 (b)
Moderators: Chem_Mod, Chem_Admin
-
Jessica Lutz 2E
- Posts: 56
- Joined: Fri Sep 29, 2017 7:04 am
Re: Problem 8.75 (b)
Drawing the lewis structures of the reactants and product helped me understand this one. When you do this, you can see more clearly that, though there are a lot of bond within each of the reactants, not all of them are broken to create the product. Looking at the lewis structure you can see the bonds that had to be broken and created to make the product and you can just add those together.
I believe that if you added the bond enthalpies of every bond in all the reactants and products you will get the same answer because the bonds that were not changed would cancel out, but that would be a lot more work.
I believe that if you added the bond enthalpies of every bond in all the reactants and products you will get the same answer because the bonds that were not changed would cancel out, but that would be a lot more work.
Re: Problem 8.75 (b)
Definitely draw out the lewis structures! It will help tremendously and if you dont it is very likely you will miss at least one bond.
-
PeterTran1C
- Posts: 30
- Joined: Thu Jul 13, 2017 3:00 am
Re: Problem 8.75 (b)
You can calculate all the bonds in both the reactants and the products and compare the difference, then calculate the bond ethalpies based off your findings.
For example: CH4 (g) + Cl2 (g) ----> CH3Cl (g) + HCl (s)
Reactants: C-H = 4 ; Cl-Cl = 1
Products: C-H = 3 ; C-Cl = 1 ; Cl-H = 1
(After you account for the differences in the bonds, you can collect the bonds that will appear in the bond enthalpy formula)
Reactants: C-H = 1 ; Cl-Cl = 1
Products: C-Cl = 1 ; Cl-H = 1
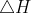 rxn = (412+242)-(338+431) = -115 kJ
rxn = (412+242)-(338+431) = -115 kJ mol-1 (the values for these bond enthalpies are found in the textbook)
mol-1 (the values for these bond enthalpies are found in the textbook)
For example: CH4 (g) + Cl2 (g) ----> CH3Cl (g) + HCl (s)
Reactants: C-H = 4 ; Cl-Cl = 1
Products: C-H = 3 ; C-Cl = 1 ; Cl-H = 1
(After you account for the differences in the bonds, you can collect the bonds that will appear in the bond enthalpy formula)
Reactants: C-H = 1 ; Cl-Cl = 1
Products: C-Cl = 1 ; Cl-H = 1
Who is online
Users browsing this forum: No registered users and 7 guests

