∆H equation
Moderators: Chem_Mod, Chem_Admin
-
Maria Bajenov 1I
- Posts: 28
- Joined: Fri Sep 29, 2017 7:06 am
∆H equation
For calculating ∆H, you solve for KJ(energy)/mol. The mol corresponds to n, but what constant corresponds to KJ?
Re: ∆H equation
If you are referring to the equation q= nCmΔT, the molar heat capacity of the substance will tell you how many joules are necessary to increase the temperature of one mol of the substance by 1 degree Celsius or Kelvin. By substituting each value into this equation, you will get a ΔH in joules per mol. To convert it to kJ per mol, you just have to divide that value by a thousand.
-
Angel Gomez 1K
- Posts: 36
- Joined: Fri Sep 29, 2017 7:04 am
Re: ∆H equation
Isn't the equation for finding change in heat as follows: 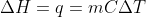
I know that the m accounts for mass instead of moles(which is usually represented as n), so would we simply switch out the m for n if we are presented with molar heat capacity?
I know that the m accounts for mass instead of moles(which is usually represented as n), so would we simply switch out the m for n if we are presented with molar heat capacity?
Re: ∆H equation
There are two different equations. The first one, ΔH = mCΔT is found using the specific heat capacity of the substance, and m stands for the mass of the substance in grams. The second one, ΔH = nCΔT is found using the molar heat capacity of the substance, and n stands for the number of mol of the substance.
Who is online
Users browsing this forum: No registered users and 15 guests

