Hess's Law Addition?
Moderators: Chem_Mod, Chem_Admin
-
LaurenDinh_3L
- Posts: 23
- Joined: Fri Sep 26, 2014 2:02 pm
Hess's Law Addition?
For a problem like 7.63, why do we need to write out all of the equations of the reactants and products? Why can't we simply use the equation: delta H rxn = sum(delta H products) - sum(delta H reactants)?
Re: Hess's Law Addition?
Krista Lum 3L
delta H rxn = sum(delta H products) - sum(delta H reactants) equation is used for the standard enthalpy formation method of determining delta H of the reaction. To solve this problem with the information given, you need to use Hess's Law in which you write out how you get the equation you need from the ones given to get credit.
delta H rxn = sum(delta H products) - sum(delta H reactants) equation is used for the standard enthalpy formation method of determining delta H of the reaction. To solve this problem with the information given, you need to use Hess's Law in which you write out how you get the equation you need from the ones given to get credit.
-
Regina Chi 2K
- Posts: 51
- Joined: Fri Sep 26, 2014 2:02 pm
Re: Hess's Law Addition?
Regardless of getting "credit" or not, I was wondering how we would know what to include in each of the steps? For example, how would we know which elements to use, what compounds are formed, and how many steps it will take?
-
Neil DSilva 1L
- Posts: 70
- Joined: Fri Sep 26, 2014 2:02 pm
Re: Hess's Law Addition?
The problem gives you the final reaction (the one we are trying to find  for). Then it gives you the
for). Then it gives you the 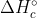 of two hydrocarbons and hydrogen gas. This value tells us the
of two hydrocarbons and hydrogen gas. This value tells us the  for the combustion reactions for each of the hydrocarbons and/or hydrogen gas. So, for example, when it gives us
for the combustion reactions for each of the hydrocarbons and/or hydrogen gas. So, for example, when it gives us 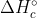 (C2H2, g), we know it means that when we combust the hydrocarbon, 1300 kj/mol is released. All combustion reactions follow the same structure: hydrocarbon reacts with oxygen to form carbon dioxide and water (in the case of the hydrogen gas, only water is formed). So if you try to write out the combustion reaction for C2H2, you'll see that it's the first reaction listed in the solution's manual. Do the same thing for the C2H6 and H2 and you'll have your three reactions. Then you just manipulate them to get the final reaction (the one mentioned in the problem), and adding the reactions together gives you the
(C2H2, g), we know it means that when we combust the hydrocarbon, 1300 kj/mol is released. All combustion reactions follow the same structure: hydrocarbon reacts with oxygen to form carbon dioxide and water (in the case of the hydrogen gas, only water is formed). So if you try to write out the combustion reaction for C2H2, you'll see that it's the first reaction listed in the solution's manual. Do the same thing for the C2H6 and H2 and you'll have your three reactions. Then you just manipulate them to get the final reaction (the one mentioned in the problem), and adding the reactions together gives you the  .
.
Who is online
Users browsing this forum: No registered users and 11 guests

