Page 1 of 1
Calculating Bond Enthalpies - #7.115
Posted: Mon Jan 19, 2015 11:08 am
by Mara Lockhart 3J
On part (b) of problem 7.115, we are asked to use bond enthalpies to calculate the enthalpy of combustion of each fuel, assuming they burn to produce gaseous CO2 and gaseous H2O. For CH4, I know we have to find all of the enthalpies and add them together, but why do the products, CO2 and H20, have negative bond enthalpies? Is this because the bonds are formed?
Re: Calculating Bond Enthalpies - #7.115
Posted: Mon Jan 19, 2015 1:15 pm
by Kayla Denton 1A
Yes! Bond forming is an exothermic process because it is the reverse of bond breaking, which is endothermic (as heat is required to break bonds).
Re: Calculating Bond Enthalpies - #7.115
Posted: Mon Jan 19, 2015 4:48 pm
by Denise 3L
For part e of this question, how did they find the heat per mole of CO2 released for each gas?
Re: Calculating Bond Enthalpies - #7.115
Posted: Mon Jan 19, 2015 4:50 pm
by Kayla Denton 1A
It's the delta H of combustion divided by the number of moles of CO2 gas produced for each substance (which can be found by the balanced equation of combustion for each substance—methane, ethanol, and octane).
Re: Calculating Bond Enthalpies - #7.115
Posted: Mon Jan 19, 2015 5:05 pm
by Denise 3L
Ahh, because the answer for octane threw me off in the solutions manual. If the delta h of combustion for octane is -5471kj/mol and there are 2 mols of C02, shouldnt it be -2735.5 k/mol CO2 instead of -684kj/mol?
Re: Calculating Bond Enthalpies - #7.115
Posted: Mon Jan 19, 2015 5:20 pm
by Neil DSilva 1L
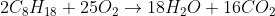
is the combustion reaction for octane, but since enthalpies are
per mole, the reaction for which enthalpy is calculated is:

. So there's 8 CO
2 produced and you would divide the enthalpy of combustion by 8 to get -684 kJ/mol CO
2 produced.
Re: Calculating Bond Enthalpies - #7.115
Posted: Mon Jan 19, 2015 8:29 pm
by Denise 3L
OH I forgot to do the balanced reaction, thank you so much!