Bond Enthalpies and Standard Enthalpies of Formation
Moderators: Chem_Mod, Chem_Admin
-
Jorja De Jesus 2C
- Posts: 121
- Joined: Sat Jul 20, 2019 12:15 am
Bond Enthalpies and Standard Enthalpies of Formation
What do you plug in to the equations for these methods if you aren't given the 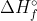 or the
or the 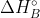 values? How do you use the equation to determine this?
values? How do you use the equation to determine this?
-
sarahsalama2E
- Posts: 164
- Joined: Fri Aug 30, 2019 12:16 am
Re: Bond Enthalpies and Standard Enthalpies of Formation
Typically, when these values are not given to you they are 0 (usually for naturally occurring compounds, such as the enthalpy of formation for O2 0). Or if no values are given to you, you may use delta G= delta H-TdeltaS
-
ValerieChavarin 4F
- Posts: 99
- Joined: Wed Sep 18, 2019 12:18 am
Re: Bond Enthalpies and Standard Enthalpies of Formation
If the values are not given, you might have to use the ∆G° = ∆H° - T∆S°equation to calculate enthalpy. If not, then the value might be zero. I don't believe there should be much confusion regarding this on the final.
Who is online
Users browsing this forum: No registered users and 13 guests

