deltaU=deltaH+w
Moderators: Chem_Mod, Chem_Admin
-
Wasila Sun 2I
- Posts: 112
- Joined: Wed Sep 30, 2020 10:00 pm
- Been upvoted: 3 times
deltaU=deltaH+w
Under what circumstances can we use the delta U=delta H+ w equation? Like what allows delta H to be interchangeable with q?
-
Justin Nguyen 3E
- Posts: 102
- Joined: Wed Sep 30, 2020 9:35 pm
Re: deltaU=deltaH+w
I think that q and deltaH can be interchangeable when the process involving these values is carried out at constant pressure.
-
Chinmayi Mutyala 3H
- Posts: 105
- Joined: Wed Sep 30, 2020 9:50 pm
-
Eliana Witham 2H
- Posts: 107
- Joined: Wed Sep 30, 2020 9:48 pm
- Been upvoted: 2 times
Re: deltaU=deltaH+w
At constant pressure, you can use deltaU = deltaH - P(deltaV) because q=deltaH and w= -P(deltaV).
-
Grecia Velasco 1G
- Posts: 131
- Joined: Thu Jul 11, 2019 12:17 am
Re: deltaU=deltaH+w
Wasila Sun 2I wrote:Under what circumstances can we use the delta U=delta H+ w equation? Like what allows delta H to be interchangeable with q?
Must be in constant pressure and a open or closed system.
-
Ethan Laureano 3H
- Posts: 102
- Joined: Wed Sep 30, 2020 9:58 pm
-
Ranen_Chang_2G
- Posts: 44
- Joined: Wed Nov 25, 2020 12:21 am
Re: deltaU=deltaH+w
The context where 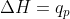 is in constant pressure. In a biological focus, we can use
is in constant pressure. In a biological focus, we can use  because reactions happen at constant pressure.
because reactions happen at constant pressure.
-
Michelle Nguyen 3F
- Posts: 104
- Joined: Wed Sep 30, 2020 10:03 pm
Re: deltaU=deltaH+w
Wasila Sun 2I wrote:Under what circumstances can we use the delta U=delta H+ w equation? Like what allows delta H to be interchangeable with q?
This can be used when the process is under constant pressure.
-
Kayla Booker 1F
- Posts: 99
- Joined: Wed Sep 30, 2020 9:43 pm
Who is online
Users browsing this forum: No registered users and 8 guests

