Relation to Internal Energy
Moderators: Chem_Mod, Chem_Admin
-
samanthaywu
- Posts: 103
- Joined: Wed Sep 30, 2020 9:51 pm
Relation to Internal Energy
I'm a little confused what the relationship between internal energy and enthalpy is. Other than delta U = q + w, what are the relationships we need to know and what conditions are those under (constant pressure, constant volume, etc.)
-
Shanna Yu 1C
- Posts: 110
- Joined: Wed Sep 30, 2020 9:36 pm
- Been upvoted: 3 times
Re: Relation to Internal Energy
Hi!
You kind of already illustrate how internal energy and enthalpy relate to each other in your question, but I can maybe rephrase it in a way where it's more obvious: H (enthalpy)= U (internal energy) + PV (work). Enthalpy is just the sum of the internal energy of the system and the product of pressure times the volume.
At constant pressure, deltaH is equal to the heat absorbed at said constant pressure. At constant volume, deltaH is equal to the internal energy of the system.
I don't think these statements are all-encompassing, so if someone wants to come by and add more, please do!
You kind of already illustrate how internal energy and enthalpy relate to each other in your question, but I can maybe rephrase it in a way where it's more obvious: H (enthalpy)= U (internal energy) + PV (work). Enthalpy is just the sum of the internal energy of the system and the product of pressure times the volume.
At constant pressure, deltaH is equal to the heat absorbed at said constant pressure. At constant volume, deltaH is equal to the internal energy of the system.
I don't think these statements are all-encompassing, so if someone wants to come by and add more, please do!
Re: Relation to Internal Energy
Basically like,,i a situation isn't doing any work, the internal energy is the same as the enthalpy, because enthalpy is the heat energy stored in a system.
-
Joshua_Chan_3K
- Posts: 46
- Joined: Wed Nov 18, 2020 12:27 am
Re: Relation to Internal Energy
Honestly U = q + w is the only equation you need to know. The others are all derived from that for instance under constant volume, no work can be done because work is distance times force so if no movement is completed, work is not done. Under those circumstances, U = q = delta H.
-
Ranen_Chang_2G
- Posts: 44
- Joined: Wed Nov 25, 2020 12:21 am
Re: Relation to Internal Energy
There was a textbook problem that really illustrates the connection between internal energy and enthalpy in constant pressure and constant volume. (4B.9). Through the equation U=q+w, w=0 because if volume is constant (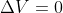 ) then no work is being done, so U=q.
) then no work is being done, so U=q.
Who is online
Users browsing this forum: No registered users and 13 guests

