Use the bond enthalpies in Tables 4E. 2 and 4E. 3 to estimate the reaction enthalpy for
a) 3 C2H2 (g) -> C6H6 (g)
I'm a little confused on how to look at the broken and formed bonds for this. I thought there were 3 broken triple C bonds, and 3 formed single C bonds, and 3 formed double bonds like 3(triple C) - [3(single C) + 3(double C)]
Textbook 4E. 5
Moderators: Chem_Mod, Chem_Admin
-
Kaitlin Eblen 1I
- Posts: 104
- Joined: Wed Feb 17, 2021 12:24 am
Re: Textbook 4E. 5
Hi Kaitlin (excellent name by the way),
Using that method I got -369 kJ. However, I saw a previous post on this question and one of the administrators answered. Since C6H6 (benzene) has 2 resonance structures, it has a special bond enthalpy (518 kJ) for these alternating double and single bonded carbons. We would multiply this value by 6 and subtract that from the positive bond enthalpies (energy required to break the bonds of the reactants). Let me know if this helps!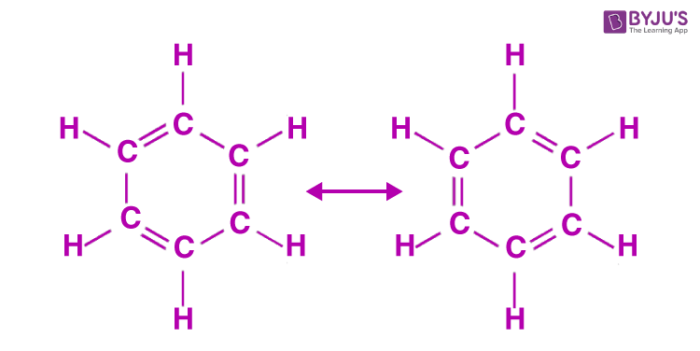
Using that method I got -369 kJ. However, I saw a previous post on this question and one of the administrators answered. Since C6H6 (benzene) has 2 resonance structures, it has a special bond enthalpy (518 kJ) for these alternating double and single bonded carbons. We would multiply this value by 6 and subtract that from the positive bond enthalpies (energy required to break the bonds of the reactants). Let me know if this helps!

-
Anna Turk 1D
- Posts: 101
- Joined: Fri Sep 24, 2021 5:14 am
Re: Textbook 4E. 5
Are we only supposed to factor in the bonds between the carbons and not between the carbons and hydrogens?
-
Arjun_Anumula_3E
- Posts: 107
- Joined: Fri Sep 24, 2021 5:47 am
- Been upvoted: 1 time
Re: Textbook 4E. 5
Since each of the 6 carbons is already attached to a hydrogen before, you don't need to worry about breaking and/or re-forming those bonds in your calculations. Drawing the structure as shown above on a problem like this definitely helps!
-
Kaitlin Joya 1I
- Posts: 124
- Joined: Fri Sep 24, 2021 7:21 am
Re: Textbook 4E. 5
Kaitlin Eblen 1I wrote:Hi Kaitlin (excellent name by the way),
Using that method I got -369 kJ. However, I saw a previous post on this question and one of the administrators answered. Since C6H6 (benzene) has 2 resonance structures, it has a special bond enthalpy (518 kJ) for these alternating double and single bonded carbons. We would multiply this value by 6 and subtract that from the positive bond enthalpies (energy required to break the bonds of the reactants). Let me know if this helps!
This did help, thank you! Does that mean we can always assume we can use the enthalpy bonds for the Kekule structure when benzene is being looked at?
-
Hannah_Pon_1F
- Posts: 103
- Joined: Fri Sep 24, 2021 6:37 am
- Been upvoted: 1 time
Re: Textbook 4E. 5
Because of the resonance of the structure, there is a special bond enthalpy that averages the single and double bonds. This is indicated in textbook by an *, so you would calculate for the formation of six of these bonds.
Who is online
Users browsing this forum: No registered users and 10 guests

