Difference between enthalpy, enthalpy of formation, and standard enthalpy of formation
Moderators: Chem_Mod, Chem_Admin
-
Nhi Pham 3D
- Posts: 40
- Joined: Mon Jan 09, 2023 9:56 am
Difference between enthalpy, enthalpy of formation, and standard enthalpy of formation
Hello! I'm a little bit confused about the difference between normal enthalpy and the enthalpy of formation. Is the enthalpy of formation just the enthalpy of a reaction that is forming/synthesizing something, or is there more to it? Also, what is the difference between the enthalpy of formation and the standard enthalpy of formation? I think the standard enthalpy of formation has to do with elements being formed from their standard states, but I also get that definition when I search up the enthalpy of formation by itself, so I'm still a little unsure. Any clarification helps - thank you!
-
Pradnya Kadam 2A
- Posts: 36
- Joined: Mon Jan 09, 2023 8:45 am
Re: Difference between enthalpy, enthalpy of formation, and standard enthalpy of formation
Hi, here are the definitions for each term:
Enthalpy: heat absorbed/released in chemical reactions or physical changes --> Symbol:
I think instead if enthalpy of formation (which I believe is the same thing as the standard enthalpy of formation) you are instead referring to the standard reaction enthalpy?
Standard Reaction Enthalpy: the reaction enthalpy when all reactants and products are in the standard state at 1 atm (for gases this would be at 1 atm and for solutions this would be 1 M AND for pure solids/liquids elements it is the most stable phase at 1 atm AND typically the temperature is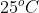 . --> Symbol:
. --> Symbol: 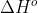
Standard Enthalpy of formation: the standard reaction enthalpy for 1 mole of a substance from its elements in their most stable form--> Symbol: . This value will always be in kilojoules per mole.
. This value will always be in kilojoules per mole.
Enthalpy: heat absorbed/released in chemical reactions or physical changes --> Symbol:
I think instead if enthalpy of formation (which I believe is the same thing as the standard enthalpy of formation) you are instead referring to the standard reaction enthalpy?
Standard Reaction Enthalpy: the reaction enthalpy when all reactants and products are in the standard state at 1 atm (for gases this would be at 1 atm and for solutions this would be 1 M AND for pure solids/liquids elements it is the most stable phase at 1 atm AND typically the temperature is
Standard Enthalpy of formation: the standard reaction enthalpy for 1 mole of a substance from its elements in their most stable form--> Symbol:
-
Nhi Pham 3D
- Posts: 40
- Joined: Mon Jan 09, 2023 9:56 am
Re: Difference between enthalpy, enthalpy of formation, and standard enthalpy of formation
Oh I see - thank you! I didn't realize that the enthalpy of formation and standard enthalpy of formation were the same thing. I can see why I got similar definitions for both terms when I searched them up. As a follow-up question, in what cases would you typically use standard enthalpy as opposed to normal enthalpy? Would it just be for the third method of calculating enthalpy where you subtract the sum of the standard enthalpies of formation for reactants from the sum of the standard enthalpies of formation for the products?
Who is online
Users browsing this forum: No registered users and 8 guests

