Hello I am on question 17 of the ThermoChem achieve assignment and I am having trouble with this problem,
A 0.357 mol sample of N2(g), initially at 298 K and 1.00 atm, is held at constant pressure while enough heat is applied to raise the temperature of the gas by 15.5 K. Calculate the amount of heat q required to bring about this temperature change, and find the corresponding total change in the internal energy delta of the gas. Assume that the constant‑pressure molar specific heat for N2(g), which consists of linear molecules, equals 7R/2, where R=8.3145 J/(mol·K) is the ideal gas constant.
I got q already by doing q=m c deltaT which is (0.357mol)(7x8.31/2)(15.5) =161.01
But for some reason, it is marking me wrong for delta U, and I don't understand why...
shouldn't q= delta U? since it is a constant pressure w=0 so, q should = delta U, right???
Achieve Weeks 3 & 4 Question #17
Moderators: Chem_Mod, Chem_Admin
-
Sam Leistiko 2B
- Posts: 50
- Joined: Mon Jan 09, 2023 8:49 am
Re: Achieve Weeks 3 & 4 Question #17
In this case, I think that you are getting confused between constant pressure and constant volume. When we talk about work for gases in a closed chamber, we talk about expansion work. In a container with a fixed volume, the gas cannot expand or contract, meaning that no work can be done on or by the system. Therefore, when you heat a gas at a constant volume, ΔU = q because w = 0. However, when you head a volume at a constant pressure, volume is not constant. Therefore, the gas is free to expand or contract, and work can be done on or by the system. Therefore, in this calculation, you need to account for the energy lost by the system as work due to expansion.
-
kirsten clemente 1E
- Posts: 45
- Joined: Mon Jan 09, 2023 2:37 am
Re: Achieve Weeks 3 & 4 Question #17
Sam Leistiko 2B wrote:However, when you heat a volume at a constant pressure, volume is not constant. Therefore, the gas is free to expand or contract, and work can be done on or by the system. Therefore, in this calculation, you need to account for the energy lost by the system as work due to expansion.
Hi, I am still confused on this part, sorry. So if the volume is allowed to expand or contract wouldn't that mean there would be more pressure in a closed system? I don't understand how if the pressure is constant if it is heated there is still work done.?
If anyone can jump in and explain further it would be great!
Sorry again.
-
Russell Ahmed 2K
- Posts: 53
- Joined: Mon Jan 09, 2023 9:33 am
Re: Achieve Weeks 3 & 4 Question #17
Since there is a change in temperature in the system (gas), there must either be an increase in pressure or increase in volume to allocate for the added heat (PV = nRT - something has to respond for this to remain true). Since the pressure is constant, the volume changes.
The volume change actually requires energy from the system itself, and that is what is represented by the work done by the system: w = -P(deltaV). So the change of internal energy delta U = q - w. The system receives heat (q) in the process of heating the gas, and it gives off energy (work) through increasing the volume of the gas sample.
Hope that helps!
The volume change actually requires energy from the system itself, and that is what is represented by the work done by the system: w = -P(deltaV). So the change of internal energy delta U = q - w. The system receives heat (q) in the process of heating the gas, and it gives off energy (work) through increasing the volume of the gas sample.
Hope that helps!
-
Omaia Olivas 1C
- Posts: 41
- Joined: Mon Jan 09, 2023 2:29 am
Re: Achieve Weeks 3 & 4 Question #17
What I did after calculating q was use the equation Cp-Cv=R to get Cv. I then inputted it into 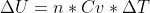 and got the right answer. I hope that helps!
and got the right answer. I hope that helps!
Re: Achieve Weeks 3 & 4 Question #17
Hi! For this question I used the q = delta H = m*C*deltaT to get solve for the q. Since this is constant pressure, q = delta H so I used q = deltaH - P*deltaV. P equals 1 (stated in the problem) and I used P*deltaV = n*R*delta T to solve for delta V. Then, I used my initial q value (which equals delta H) and delta V value in the delta U = delta H - P*delta V equation to get my delta U. Sorry if this types out didn't make sense!
Return to “Heat Capacities, Calorimeters & Calorimetry Calculations”
Who is online
Users browsing this forum: No registered users and 12 guests

