An 80.0 g sample of a gas was heated from 25 C to 225C. During this process, 346 J of work was done by the system and its internal energy increased by 8245 J. What is the specific heat of the gas?
What is "q" within this or how do you calculate "q"?
Sapling Question
Moderators: Chem_Mod, Chem_Admin
-
Amanda Tran 1D
- Posts: 101
- Joined: Fri Sep 24, 2021 7:10 am
- Been upvoted: 1 time
Re: Sapling Question
We know that delta U = q + w. You're given w in the problem and delta U, so you can solve for the value of q. Once you get that, you can use 1=nCdelta T to find the specific heat of the gas.
-
joselle barnoya 1k
- Posts: 24
- Joined: Mon Jan 09, 2023 8:37 am
Re: Sapling Question
As the previous answerer said, you plug in 8245J as U and 346J as w, then you solve for q. Once you have calculated q (I got 7899J) you plug it into the equation 
, where Cp is the specific heat of the gas.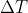 is Tfinal - T initial which is 200 in this case. m refers to the mass of the sample. While
is Tfinal - T initial which is 200 in this case. m refers to the mass of the sample. While  is also an existing formula, since we don't know the molar mass of the sample we can't use this equation (we don't know n). For our purposes, the only difference between either of these equations is that our C with the n equation is the molar heat and the C from the m equation is the specific heat.
is also an existing formula, since we don't know the molar mass of the sample we can't use this equation (we don't know n). For our purposes, the only difference between either of these equations is that our C with the n equation is the molar heat and the C from the m equation is the specific heat.
, where Cp is the specific heat of the gas.
Re: Sapling Question
Because you have both the delta U values and the work done on the system, plug the values into delta U = q + w and solve to find the q value! Remember the q value is the net heat transferred into the system!
Return to “Heat Capacities, Calorimeters & Calorimetry Calculations”
Who is online
Users browsing this forum: No registered users and 7 guests

