Chapter 8 Problem 31
Moderators: Chem_Mod, Chem_Admin
-
Kendall_Chaffin_3C
- Posts: 25
- Joined: Wed Sep 21, 2016 2:59 pm
Chapter 8 Problem 31
In the solution for problem 31 of chapter 8 it says the molar heat capacity of a monatomic ideal gas at constant pressure is C=5/2R and the molar heat capacity of a monatomic ideal gas at constant volume is C=3/2R. Where did the numbers 3/2 and 5/2 come from? Are these statements true for any problem?
-
E_Villavicencio 2N
- Posts: 26
- Joined: Wed Sep 21, 2016 2:59 pm
Re: Chapter 8 Problem 31
These values come from the equipartition theorem, which is used to estimate the translational (energy due to motion through space) and rotational (energy due to the rotational motion of a molecule) contributions to the internal energy of an ideal gas.
The book doesn't derive the main equation of the equipartition theorem which is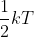 (k is the Boltzmann's constant and T is temperature). Using that equation and the heat capacity equation (
(k is the Boltzmann's constant and T is temperature). Using that equation and the heat capacity equation ( ), it can be said that the molar heat capacity of a monoatomic ideal gas at constant volume is
), it can be said that the molar heat capacity of a monoatomic ideal gas at constant volume is  , and the molar heat capacity of a linear molecule at constant pressure is
, and the molar heat capacity of a linear molecule at constant pressure is 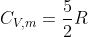 .
.
There's also a value for nonlinear molecules, and the values for these three types (monoatomic gases, linear, and nonlinear molecules) in the case of constant pressure. You can check all these values in page 281.
The book doesn't derive the main equation of the equipartition theorem which is
There's also a value for nonlinear molecules, and the values for these three types (monoatomic gases, linear, and nonlinear molecules) in the case of constant pressure. You can check all these values in page 281.
Return to “Heat Capacities, Calorimeters & Calorimetry Calculations”
Who is online
Users browsing this forum: No registered users and 4 guests

