Difference between Delta H and q?
Moderators: Chem_Mod, Chem_Admin
-
jonathanshi_1A
- Posts: 22
- Joined: Fri Jul 22, 2016 3:00 am
Difference between Delta H and q?
I'm a little confused as to the difference between the two, as they both have to do with heat.
-
Rachel_Prescott_2M
- Posts: 10
- Joined: Wed Sep 21, 2016 2:57 pm
- Been upvoted: 1 time
Re: Difference between Delta H and q?
q is the amount of heat transferred to a system whereas  is used to describe the change in enthalpy. Enthalpy is the total potential energy of a system, which is associated with the heat transferred to/from a system (q). However, at constant pressure
is used to describe the change in enthalpy. Enthalpy is the total potential energy of a system, which is associated with the heat transferred to/from a system (q). However, at constant pressure 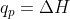 which can make it difficult to see the difference between what the two values are. Hope this helped a bit!
which can make it difficult to see the difference between what the two values are. Hope this helped a bit!
-
jeree pucan
- Posts: 20
- Joined: Wed Nov 18, 2015 3:00 am
Re: Difference between Delta H and q?
I guess in simpler terms you can say q = heat in general, whereas H (enthalpy) = qp which is heat at a constant pressure.
-
Tycho_Meimban_2B
- Posts: 31
- Joined: Wed Sep 21, 2016 2:57 pm
Re: Difference between Delta H and q?
Q is the energy transfer due to thermal reactions such as heating water, cooking, etc. anywhere where there is a heat transfer. You can say that Q (Heat) is energy in transit.
Enthalpy (Delta H), on the other hand, is the state of the system, the total heat content.
They both can deal with heat (qp) (Q at constant pressure) = (Delta H) but both Heat and Enthalpy always refer to energy, not specifically Heat.
Hope this helps!
Enthalpy (Delta H), on the other hand, is the state of the system, the total heat content.
They both can deal with heat (qp) (Q at constant pressure) = (Delta H) but both Heat and Enthalpy always refer to energy, not specifically Heat.
Hope this helps!
Return to “Heat Capacities, Calorimeters & Calorimetry Calculations”
Who is online
Users browsing this forum: No registered users and 3 guests

