4C.3 part B (7th edition) - relating qv to delta H
Moderators: Chem_Mod, Chem_Admin
-
Andie Jian 1D
- Posts: 69
- Joined: Fri Sep 28, 2018 12:17 am
- Been upvoted: 1 time
4C.3 part B (7th edition) - relating qv to delta H
In this problem we need to calculate the final temperature and change in enthalpy when 765J of heat are transferred to .820 mol Kr(g) at 298K and 1.00atm. In part, b, you are supposed to do both of these things at a constant volume. I understand that you can find qv and relate it to change in internal energy and find the final temperature from qv. However, I am confused how we are supposed to relate these variables at constant volume to change in enthalpy. The book gives the equation 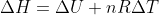 , but I don't think we ever learned this equation in class and it is not on the constants and equations sheet. Are we expected to memorize this?
, but I don't think we ever learned this equation in class and it is not on the constants and equations sheet. Are we expected to memorize this?
-
aisteles1G
- Posts: 117
- Joined: Fri Sep 28, 2018 12:15 am
Re: 4C.3 part B (7th edition) - relating qv to delta H
The equation is a derivation, w=P*V and P*V=nRT (ideal gas law) but this time having the change in temp included, and H=U+w, so I think the book expects you to make the connection but honestly I think it would be better just to have it memorized. The book chapter 4C does explain this equation and it says it's always valid at constant volume. hope this helps!
Return to “Heat Capacities, Calorimeters & Calorimetry Calculations”
Who is online
Users browsing this forum: No registered users and 5 guests

