Equations for work, internal energy, and heat
Moderators: Chem_Mod, Chem_Admin
-
Jackson Crist 1G
- Posts: 67
- Joined: Mon Jan 09, 2023 2:47 am
Equations for work, internal energy, and heat
I'm doing the achieve assignment for week 3-4 and im struggling with almmost every question because they change the equations every time. I read the hint and every single time, the equation given as a hint for either work, internal energy, or heat is totally different than the last question! Can someone tell me the legitimate equations for internal energy, work, and heat, because the achieve has given me multiple different ones for each (not to mention how these equations are different than the ones given in class).
-
Bridget Vause 1A
- Posts: 35
- Joined: Mon Jan 09, 2023 2:19 am
Re: Equations for work, internal energy, and heat
Hi!
So we learned in class today that the internal energy equation is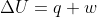 . q is the heat (amount the the system is heated/cooled) and w is the work (amount the system is being expanded or compressed).
. q is the heat (amount the the system is heated/cooled) and w is the work (amount the system is being expanded or compressed).
The equation for work is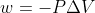 . (P is the external pressure and delta V is the change in volume).
. (P is the external pressure and delta V is the change in volume).
Hope this helps! :)
So we learned in class today that the internal energy equation is
The equation for work is
Hope this helps! :)
-
Lizze Cardoso 2I
- Posts: 34
- Joined: Mon Jan 09, 2023 9:24 am
Re: Equations for work, internal energy, and heat
Hi! I also had difficulty working through the assignment, given that the hint provided different equations every time for solving the problem. However, as we learned in the lecture today, we can use other equations for the variable listed in the equation and substitute them for solving for internal energy. It depends on what information and variables we are given and rearranging them to help us solve what the question is asking.
Return to “Heat Capacities, Calorimeters & Calorimetry Calculations”
Who is online
Users browsing this forum: No registered users and 7 guests

