An ice cube with a mass of 46.2 g at 0.0
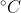
is added to a glass containing 396 g of water at 45.0
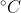
. Determine the final temperature of the system at equilibrium. The specific heat capacity of water, Cs, is 4.184 J/g⋅
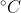
, and the standard enthalpy of fusion,

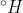
fus, of water is 6.01×103 J/mol. Assume that no energy is transferred to or from the surroundings.
I am plugging the numbers into the equation

H

fus + q ice = -q water but I got 0.0256 which doesn't make sense. Please help.
Thanks!!