Angstrom units
Moderators: Chem_Mod, Chem_Admin
-
Megan McKenna Dis1A
- Posts: 101
- Joined: Fri Sep 24, 2021 6:17 am
Angstrom units
What is the purpose of using the Angstrom units and how to I know when to apply them?
-
Ryan Burchell 3D
- Posts: 59
- Joined: Fri Sep 24, 2021 7:11 am
Re: Angstrom units
An angstrom is 10^-10 of a meter and it is just a convenient name for around the size of a atoms. Just easier to say "this atom is 3 angstroms" instead of "3^-10 meters"
-
Eszter Kovacs 1A
- Posts: 100
- Joined: Fri Sep 24, 2021 5:41 am
Re: Angstrom units
Hi, I think it just makes it easier to describe the length of a wavelength, especially if the wavelength is really short. You can probably just use meters unless the exercise specifically asks for angstrom.
-
Kainath Kamil Dis 2K
- Posts: 103
- Joined: Fri Sep 24, 2021 5:37 am
Re: Angstrom units
Wavelengths can get very small, so an Angstrom is 10^-10 m and we just say that instead of "meters" sometimes.
-
Aparna Pillai 1E
- Posts: 109
- Joined: Fri Sep 24, 2021 7:09 am
Re: Angstrom units
One angstrom (Å) is equivalent to 10^-10 meters. Although it is not an SI unit, it is useful for expressing atomic dimensions. A reason for this is that the diameter of an atom is on the order of angstroms.
-
Daniela G 2C
- Posts: 92
- Joined: Fri Sep 24, 2021 5:06 am
Re: Angstrom units
Angstrom units can describe very small lengths such as quantities like atoms and molecules. Instead of an extremely small meter unit, we can use Angstrom units.
1m = 10^10A
1A = 10^-10m
1m = 10^10A
1A = 10^-10m
-
Daniel Li 3C
- Posts: 102
- Joined: Fri Sep 24, 2021 5:12 am
Re: Angstrom units
I think that it is used to measure really small wavelengths or atoms because it is 10^-10, I believe it is named after a 19th century Swedish physicist
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Angstrom units
Hello Megan,
The Angstrom unit ( ) is commonly used by chemists in terms of describing bond length, the distance between the nuclei of two adjacent atoms.
) is commonly used by chemists in terms of describing bond length, the distance between the nuclei of two adjacent atoms.
It is simply more convenient to have the unit as it provides "nice" numbers, such as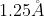 for a bond length.
for a bond length.
The Angstrom unit (
It is simply more convenient to have the unit as it provides "nice" numbers, such as
Re: Angstrom units
I would say that the whole point of using Angstrom units is to describe the length of the wavelengths with easier and more simpler numbers.
-
Matthew Vu 3C
- Posts: 109
- Joined: Fri Sep 24, 2021 5:07 am
- Been upvoted: 1 time
Re: Angstrom units
Angstroms are usually used when discussing wavelengths of light. In all of the light equations, Angstrom isn't the SI unit, so you probably should only convert to angstroms if it asks you do to that in your final answer.
-
Nicola Higgins 14B
- Posts: 103
- Joined: Fri Sep 24, 2021 5:41 am
Re: Angstrom units
Angstrom units are represented by the A with the small circle attached to the top of the A, and is just another conversion factor we should know how to use. It's conversion is on the cheat sheet, though! :)
-
Kainath Kamil Dis 2K
- Posts: 103
- Joined: Fri Sep 24, 2021 5:37 am
Re: Angstrom units
Angstroms are 10^-10 usually to measure wavelengths because wavelengths can get really small.
-
Litsa Dimit 1D
- Posts: 84
- Joined: Fri Sep 24, 2021 5:27 am
Re: Angstrom units
It just helps the questions ask us to use angstrom units since its 10^-10 meters. It is a conversion, also on the cheat sheet!
-
Akshat Katoch 2K
- Posts: 95
- Joined: Fri Sep 24, 2021 5:41 am
Re: Angstrom units
An Angstrom is 10^-10 meters. Since wavelengths can get very small it is a good unit to use to describe its size. We just say angstrom because it's a lot easier to describe and math than if we used meter units.
Re: Angstrom units
Angstrom units are mainly used to describe a wavelength. It can also be used to describe a chemical bond length. Any length can be converted to Angstrom units. However, it is mainly used to describe very small things because Angstrom is 10^-10m.
-
Cynthia_L_2C
- Posts: 99
- Joined: Fri Sep 24, 2021 7:25 am
Re: Angstrom units
Angstrom units are mainly used when describing wavelength, or used when describing chemical bond length. Since it is 10^-10m, it is used to decribe smaller numbers.
-
trevina_brown_2A
- Posts: 105
- Joined: Wed Feb 03, 2021 12:15 am
-
Angela Harrington 2L
- Posts: 144
- Joined: Fri Sep 24, 2021 5:22 am
Re: Angstrom units
A single angstrom (Å) is equivalent to 10^-10 meters, and I'm pretty sure it's just a more efficient unit to use when describing say the size of a wavelength because they can be so small.
-
Joseph Ettipio
- Posts: 101
- Joined: Fri Sep 24, 2021 5:27 am
Re: Angstrom units
I believe Angstroms are also convenient because single digit measures of them can describe the length of bonds between atoms. Most chemical bonds are generally in the range of 1-2 Angstroms.
-
Colby Irvine 2A
- Posts: 49
- Joined: Fri Sep 24, 2021 5:25 am
Re: Angstrom units
Typically chemical bonds are between 1-2 Angstroms which makes it a convenient notation.
-
Sneha Anantharaman2D
- Posts: 101
- Joined: Fri Sep 24, 2021 5:35 am
- Been upvoted: 2 times
Re: Angstrom units
I believe Angstroms are simply much more efficient to use when looking at bond lengths or atomic radii, since one Angstrom is equal to 10^-10m.
-
Sonia Virk 2A
- Posts: 51
- Joined: Fri Sep 24, 2021 5:13 am
Re: Angstrom units
Angstrom is just another name for 10^-10m which is convenient because the names of numbers can get quite complicated when they are that small. Hope this helps!
Re: Angstrom units
it is used for sizes of atoms, molecules, lengths of chemical bonds, and for wavelengths. It is 10^-10 (0.1 nanometers).
Return to “SI Units, Unit Conversions”
Who is online
Users browsing this forum: No registered users and 5 guests

