Page 1 of 1
Avogadro's Number
Posted: Sun Apr 15, 2018 7:32 pm
by Kate Manganaro 1F
So is Avogadro's Number used only when converting from moles to atoms or can it be used to convert from moles to molecules as well?
Re: Avogadro's Number
Posted: Sun Apr 15, 2018 7:36 pm
by Heung Ching Chia 1E
I believe avogadro's number can be used to convert both from moles to atoms and from moles to molecules.
Re: Avogadro's Number
Posted: Sun Apr 15, 2018 7:44 pm
by octaviahuang1f
Yes, it can be used to convert from moles to molecules as well (1 mole= 6.022 × 10^23 molecules). I think it depends on the basic units of the substance; whether it's atoms or molecules.
Re: Avogadro's Number [ENDORSED]
Posted: Sun Apr 15, 2018 8:06 pm
by tmehrazar
Yes it can be used for both and it does depend on the basic units of the substance.
Re: Avogadro's Number
Posted: Sun Apr 15, 2018 9:49 pm
by Mohamad 1J
Hi Kate,
Avagadros number is used to find the amount of blank in blank. It can be applied to the number of atoms in an element, the number of molecules in a compound, and even the amount of formula units in an ionic compound.
Re: Avogadro's Number
Posted: Sun Apr 15, 2018 10:10 pm
by Luis Torres 1C
It can be used both ways, as long as it is applied correctly (multiplying by it when going from moles to atoms and dividing when going from atoms to moles)
Re: Avogadro's Number
Posted: Sat Oct 06, 2018 12:23 pm
by Jchellis 1I
So when asked for atoms of a molecule we can multiply by the mols by Avogadro's number to get number of atoms, but I do not understand how to get molecules from mols?
Re: Avogadro's Number
Posted: Sat Oct 06, 2018 12:51 pm
by 904936893
Jchellis 1I wrote:So when asked for atoms of a molecule we can multiply by the mols by Avogadro's number to get number of atoms, but I do not understand how to get molecules from mols?
You can do the same thing to get molecules, because moles is just a unit of "something" not just an atom, but also molecules or formula units. So you would multiply the amount of moles of the molecule you are looking at by Avogadro's number, to get the number of molecules.
Re: Avogadro's Number
Posted: Sun Oct 07, 2018 5:01 pm
by Shibhon_Shepard
would you use the inverse if you're going in the opposite direction?
can someone clarify what would it be for :
- atoms to moles
- moles to atoms
thank you
Re: Avogadro's Number
Posted: Sun Oct 07, 2018 5:09 pm
by Jeremy_Guiman2E
Yes, because Avogadro's number is used as a conversion factor, it is reversible.
So, going from "x" atoms to moles, you would say "x" atoms times (1 mol/6.022 x 1023 atoms) = y mol.
Let's say you have 15 atoms. 15 atoms x (1 mol/6.022 x 1023 atoms) = 2.5 x 10-23 mol.
Alternatively, going from "x" moles to atoms, you would say "x" moles" times (6.022 x 1023 atoms/1 mol) = y atoms.
So here, as an example, we might have 11 mol. 11 mol x (6.022 x 1023 atoms/1 mol) = 6.6 x 1024 atoms.
To sum again, you can use the inverse to solve a problem, just make sure there is cancellation of units.
Re: Avogadro's Number
Posted: Sun Oct 07, 2018 5:25 pm
by Chem_Mod
Suppose you want to convert 3 moles of O to atoms.

Likewise, if you want to convert 1.806*10^{24}[/tex] atoms to moles, you do the opposite.
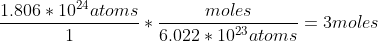
You could look at it as the inverse to go in either direction, but it may be simpler to think of using Avogadro's number to cancel out units to convert into another set of units.
Re: Avogadro's Number
Posted: Tue Oct 09, 2018 5:25 pm
by Nicolette_Canlian_2L
Does Avogadro's number include units, or do we use the number alone?