Quiz 1
Moderators: Chem_Mod, Chem_Admin
-
Dennisse Diaz 1D
- Posts: 33
- Joined: Mon Apr 09, 2018 1:39 pm
- Been upvoted: 1 time
Quiz 1
I am confused as to how to tell the difference between the empirical formula and the molecular formula... There was a question on the exam that gave multiple molecules and we had to give both the empirical and molecular formula, how do we find them? Someone help :(
-
sharonvivianv
- Posts: 63
- Joined: Fri Apr 06, 2018 11:05 am
Re: Quiz 1
It's easier if you find the molecular formula first. Once you find it, you can try to divide it by the least common denominator that will make it the empirical formula. Sometimes the molecular and empirical formulas are the same. Remember that the molecular formula gives the actual number and the empirical formula gives a ratio.
-
Kara Justeson 1B
- Posts: 31
- Joined: Fri Apr 06, 2018 11:03 am
Re: Quiz 1
The empirical formula would be the smallest ratio of the elements. For example if the molecular formula was C4H10, the empirical formula would be C2H5 (divided by a ratio of 2). Whereas the molecular formula tells you how many atoms are in that compound.
-
Rebecca Chu 1C
- Posts: 27
- Joined: Fri Apr 06, 2018 11:02 am
Re: Quiz 1
The empirical formula expresses the atoms in a molecule/compound in terms of ratios. The molecular formula shows the actual number of atoms in a molecule/compound. Some molecules can have the same empirical formula (e.g. glucose and acetic acid both have the empirical formula of  but glucose has the molecular formula
but glucose has the molecular formula 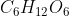 and acetic acid
and acetic acid 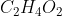 )
)
-
Dennisse Diaz 1D
- Posts: 33
- Joined: Mon Apr 09, 2018 1:39 pm
- Been upvoted: 1 time
Re: Quiz 1
Kara Justeson 1B wrote:The empirical formula would be the smallest ratio of the elements. For example if the molecular formula was C4H10, the empirical formula would be C2H5 (divided by a ratio of 2). Whereas the molecular formula tells you how many atoms are in that compound.
-This was very helpful!! does this mean we have to find the molecular formula and once we find that we can reduce it to its lowest value and that would give us the empirical formula?
Re: Quiz 1
yes if you found the molecular formula all you have to do would be to reduce it to its lowest form, with all the numbers being whole numbers and you would have the empirical formula
-
fara valdez
- Posts: 48
- Joined: Fri Apr 06, 2018 11:05 am
Re: Quiz 1
I was also confused. I think that there should have been some sort of elaboration on it, or at least a clear demonstration of it.
Return to “SI Units, Unit Conversions”
Who is online
Users browsing this forum: No registered users and 3 guests

