Hello! I can't seem to get the right answer to this question. Could someone help explain how they solved?
You use an electron microscope in which the matter-wave associated with the electron beam has a wavelength of 0.0431 nm. What is the kinetic energy of an electron in the beam, expressed in electron volts?
Sapling WEEK 4 #22
Moderators: Chem_Mod, Chem_Admin
-
Kaylee Messick 3J
- Posts: 102
- Joined: Wed Sep 30, 2020 9:54 pm
Re: Sapling WEEK 4 #22
Hello! For this problem, you would use the wavelength provided to find the velocity using the de Broglie equation. Then you would use this velocity to find the kinetic energy in joules, and convert this answer to eV. Hope this helps!
-
Valerie Doan 3I
- Posts: 115
- Joined: Wed Sep 30, 2020 10:03 pm
- Been upvoted: 1 time
Re: Sapling WEEK 4 #22
First, you use De Broglie's equation 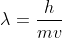 but re-arrange it to find velocity instead. So that would be
but re-arrange it to find velocity instead. So that would be 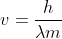 . Using that velocity, you plug it into the kinetic energy equation
. Using that velocity, you plug it into the kinetic energy equation 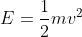 . Your answer will be in Joules but you need to convert it to electron volts so divide E by
. Your answer will be in Joules but you need to convert it to electron volts so divide E by 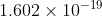 .
.
-
Sarah Huang 3A
- Posts: 70
- Joined: Wed Sep 30, 2020 9:48 pm
- Been upvoted: 1 time
Re: Sapling WEEK 4 #22
Melanie Krahn 1C wrote:Hello! I can't seem to get the right answer to this question. Could someone help explain how they solved?
You use an electron microscope in which the matter-wave associated with the electron beam has a wavelength of 0.0431 nm. What is the kinetic energy of an electron in the beam, expressed in electron volts?
Hi Melanie! In order to find the kinetic energy in electrovolts,you first need to convert the wavelength into the energy using E = hv. But since you have a wavelength, you convert the equation to E = (hc)/lambda, the lambda being the wavelength gave you. They gave you 0.0431 nm, so you convert that to meters. Since it is in nanometers, you know that nano indicates 10^-9, so you wavelength is 0.0431 x 10^-9m.
Using that, your current equation is now E = ((6.626*10^/34J*s)(2.998*10^8 m/s))/ (0.0431 x 10^-9m). Solve that equation, make sure to check out your units, and then once you get your answer, which is in Joules, you convert it to electrovolts (eV).
In order to do that, you use the conversion factor 1 eV = 1.602 * 10^-19 J.
I hope that helps!
My way isn't necessarily the right way though since I use the equations for a photon, but you can definitely use the de broglie wavelenth to figure it out too! Just remember to use your conversions and check your units.
-
reva_bajjuri
- Posts: 117
- Joined: Fri Oct 02, 2020 12:17 am
Re: Sapling WEEK 4 #22
for this problem, would you use the 4 sig figs in the conversion from joules to electo volts or are those not significant figures?
-
Britney Tran IJ
- Posts: 103
- Joined: Fri Aug 30, 2019 12:18 am
Re: Sapling WEEK 4 #22
reva_bajjuri wrote:for this problem, would you use the 4 sig figs in the conversion from joules to electo volts or are those not significant figures?
I believe you would use the sig figs from the value given in the problem (0.0431). Therefore, your answer should have 3 sig figs.
Return to “SI Units, Unit Conversions”
Who is online
Users browsing this forum: No registered users and 8 guests

