I know that alkanes have the general molecular formula:
Molecular Formula and Chemical Structure
Moderators: Chem_Mod, Chem_Admin
-
Michelle_Nguyen_3F
- Posts: 40
- Joined: Wed Sep 21, 2016 2:59 pm
Molecular Formula and Chemical Structure
Hello!
I know that alkanes have the general molecular formula: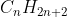 and that any compound with this carbon and hydrogen ration must be an alkane. In the course reader, it asks if we are able to determine the structure from the molecular formula. It then says we do not know the structure from the molecular formula if there are 4 or more carbons. Why is this the case? Thank you!
and that any compound with this carbon and hydrogen ration must be an alkane. In the course reader, it asks if we are able to determine the structure from the molecular formula. It then says we do not know the structure from the molecular formula if there are 4 or more carbons. Why is this the case? Thank you!
I know that alkanes have the general molecular formula:
-
mikezargari
- Posts: 20
- Joined: Wed Sep 21, 2016 2:56 pm
- Been upvoted: 1 time
Re: Molecular Formula and Chemical Structure
When dealing with molecules that have four or less carbons the chemical structure can only be unbranched in order to accommodate all carbons and hydrogens in the molecule. When a molecule contains 5 or more carbons the molecule's structure can begin to take various forms. Each of these forms are equally valid structures, which prevents us from being able to know which one the molecular formula is intending to represent. Hope this helped.
-
Shadi_Keyvani_1D
- Posts: 27
- Joined: Wed Sep 21, 2016 2:56 pm
Re: Molecular Formula and Chemical Structure
This is saying that we don't know the specific structure of compounds with more than 4 carbon atoms due to the existence of constitutional isomers b/c of different unbranched/branched structures.
Who is online
Users browsing this forum: No registered users and 3 guests

