Line Structures of Alkynes
Moderators: Chem_Mod, Chem_Admin
-
Hank Wang 2J
- Posts: 25
- Joined: Fri Sep 25, 2015 3:00 am
Line Structures of Alkynes
So I noticed that the alkanes and alkenes line structures are always bent indicating a carbon, but for alkynes in the homework it is always straight. I was just wondering if it would be considered wrong to draw it like an alkane or alkene but still indicating the triple bond?
-
fatima donato 1H
- Posts: 11
- Joined: Tue Nov 25, 2014 3:00 am
Re: Line Structures of Alkynes
On page 20 of the Introduction to Organic Chemistry text book we are told that triple bonds consist of one sigma bond and 2 pi bonds, which produce a linear molecule (bond angle is 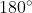 ). With that being said, I think drawing alkynes as linear (straight) structures would be correct. Follow the examples in the book. I hope this helps!
). With that being said, I think drawing alkynes as linear (straight) structures would be correct. Follow the examples in the book. I hope this helps!
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Line Structures of Alkynes
This is correct, line structures are drawn to invoke actual geometry, and because alkynes are linear, their line structures should be as well.
Who is online
Users browsing this forum: No registered users and 3 guests

