Homework Ch. 2 #30
Moderators: Chem_Mod, Chem_Admin
-
Michelle_Nguyen_3F
- Posts: 40
- Joined: Wed Sep 21, 2016 2:59 pm
Homework Ch. 2 #30
Could someone describe/break down their thought process in writing the IUPAC and the common name for 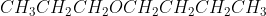 ? Thank you!
? Thank you!
-
shreya_mantri_3I
- Posts: 17
- Joined: Fri Jun 17, 2016 11:28 am
Re: Homework Ch. 2 #30
This is the structural formula of propoxybutane, propoxy comes before butane because the functional group is attached to a shorter chain and is treated a substituent. The longest alkane chain forms the root of the name.
-
regina_ho_3A
- Posts: 13
- Joined: Fri Jul 15, 2016 3:00 am
Re: Homework Ch. 2 #30
I would first draw out the molecule so I can see how the oxygen is attached to the carbons. After drawing it out, you should be able to clearly see that there is an ether functional group. We know that in order to name an ether, we have to identify the longest chain and the shortest chain. The shortest chain gets the suffix dropped and -oxy added to its name. In this case the shortest chain has the prefix prop- so, this part should be propoxy. The longest chain has 4 carbons and is kept the same, so it is butane. The ether functional group is attached to the first carbon on the longest chain. So the IUPAC name should be 1-propoxybutane.
Re: Homework Ch. 2 #30
Also, for the final will we have to know both the common names and IUPAC names or can we just know the IUPAC names?
Who is online
Users browsing this forum: No registered users and 4 guests

