Charges on Amines
Moderators: Chem_Mod, Chem_Admin
-
Tasnim Sazzad 4B
- Posts: 24
- Joined: Fri Sep 25, 2015 3:00 am
Charges on Amines
Is there supposed to be a charge on amines? Because wouldn't a tertiary amine have one more bond the the first three types?
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Charges on Amines
To be neutral, nitrogen should have three bonds and one lone pair. A primary amine has two bonds to hydrogens, one bond to an alkyl group, and one lone pair (example: 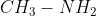 . A secondary amine has one bond to hydrogen, two bonds to alkyl groups, and one lone pair (example:
. A secondary amine has one bond to hydrogen, two bonds to alkyl groups, and one lone pair (example: 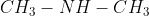 . A tertiary amine has three bonds to alkyl groups and one lone pair (example:
. A tertiary amine has three bonds to alkyl groups and one lone pair (example: _{3}-N) . In all of these, the nitrogen has three bonds and one lone pair, giving it a neutral charge.
. In all of these, the nitrogen has three bonds and one lone pair, giving it a neutral charge.
-
parisnortega1
- Posts: 34
- Joined: Mon Jan 09, 2023 2:29 am
Re: Charges on Amines
In amines, nitrogen is bonded to three other atoms (alkyl groups or hydrogens) and has a lone pair of nonbonding electrons. This lone pair produces a region of electron density on the nitrogen atom, which coupled with the electronegativity of nitrogen produces a partial-negative charge at that location.
Who is online
Users browsing this forum: No registered users and 4 guests

