Delta H
Moderators: Chem_Mod, Chem_Admin
-
JacksonWissing2G
- Posts: 103
- Joined: Fri Sep 24, 2021 5:43 am
- Been upvoted: 1 time
Re: Delta H
Spontaneity is determined by the delta G, with a positive delta g being not spontaneous and a negative one being spontaneous. We can determine what it is by using the equation ΔG=ΔH−TΔS, and since delta H is usually larger than T delta S, a negative delta H usually means the reaction will be spontaneous.
-
Xuan Lai 1H
- Posts: 100
- Joined: Fri Sep 24, 2021 5:13 am
- Been upvoted: 4 times
Re: Delta H
While a reaction with a negative delta H is likely to be spontaneous, it is not always the case, so we must look at the change in Gibbs free energy (Delta G):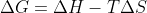 . If delta H is negative, and delta S is positive, the resulting value will be a negative number (by just looking at the signs) so it would be spontaneous at all temperatures (-H - T*+S = -G). On the other hand, if delta H was positive, then the reaction could, or could not be, spontaneous depending on whether the delta S was positive/negative and greater than H.
. If delta H is negative, and delta S is positive, the resulting value will be a negative number (by just looking at the signs) so it would be spontaneous at all temperatures (-H - T*+S = -G). On the other hand, if delta H was positive, then the reaction could, or could not be, spontaneous depending on whether the delta S was positive/negative and greater than H.
-
Jeffrey Yang 3I
- Posts: 50
- Joined: Mon Jan 03, 2022 9:37 pm
Re: Delta H
Delta H as a negative value means that the reaction is exothermic. This implies that the reaction releases heat, or the overall energy of the system increases. However, this does not correlate to spontaneity when considering other factors such as activation energy and gibbs free energy.
-
Kiku Shirakata 2A
- Posts: 103
- Joined: Fri Sep 24, 2021 5:11 am
Re: Delta H
Hello,
I think Question #12 on Week 5 & 6 is a great reference to better understand this concept. We know that delta G must be negative for a spontaneous process since that means delta S of the universe if positive. By using the equation, delta G = delta H - T*delta S, we can see what values plugged in can result in a negative delta G, which is the indicator of a spontaneous process. When delta H is negative and delta S is positive, the process is always spontaneous and when delta H is negative and delta S is negative, the process is spontaneous below a certain temperature. On the other hand, when delta H is positive and delta S is negative, the reverse process is always spontaneous and when delta H is positive and delta S is positive, the process is spontaneous above a certain temperature. Thus, we can see that negative delta H gives us a much more likely chance of the forward process to be a spontaneous one.
Hope this helps!
I think Question #12 on Week 5 & 6 is a great reference to better understand this concept. We know that delta G must be negative for a spontaneous process since that means delta S of the universe if positive. By using the equation, delta G = delta H - T*delta S, we can see what values plugged in can result in a negative delta G, which is the indicator of a spontaneous process. When delta H is negative and delta S is positive, the process is always spontaneous and when delta H is negative and delta S is negative, the process is spontaneous below a certain temperature. On the other hand, when delta H is positive and delta S is negative, the reverse process is always spontaneous and when delta H is positive and delta S is positive, the process is spontaneous above a certain temperature. Thus, we can see that negative delta H gives us a much more likely chance of the forward process to be a spontaneous one.
Hope this helps!
-
Hannah Jin 1J
- Posts: 102
- Joined: Fri Sep 24, 2021 6:42 am
Re: Delta H
A negative delta H indicates an exothermic process which is favorable because it releases rather than requires heat as the reaction proceeds. This doesn't mean the reaction will automatically be spontaneous because entropy also plays a factor into delta G, however, a delta H helps make the delta G more negative/spontaneous.
-
Jennifer Fuentes 2K
- Posts: 99
- Joined: Fri Sep 24, 2021 6:17 am
Re: Delta H
A spontaneous reaction will always occur when Delta H is negative and Delta S is positive, and a reaction will always be non-spontaneous when Delta H is positive and Delta S is negative.
Who is online
Users browsing this forum: No registered users and 3 guests

