A cotton ball soaked in KCL is lit on fire. The flame is a violet color (λ=400 nm). A scientist claims that the color arises from the transition of an electron from its excited state to its ground state. What is the difference in energy between these two states?
I know I use the equation E=hc/λ but I still am unable to get the correct answer so if someone could help it would be greatly appreciated.
Test 2 Question 4
Moderators: Chem_Mod, Chem_Admin
-
Chris Qiu 1H
- Posts: 31
- Joined: Fri Apr 06, 2018 11:04 am
Re: Test 2 Question 4
you would have to use the reinberg equation with a Balmer series drop (you would know you need to see this since the wavelength is in the visible range which is the balmer series) a balmer series is a drop from n=x to n=2
-
Liliana Rosales 1E
- Posts: 36
- Joined: Wed Nov 15, 2017 3:03 am
-
Jimmy lira-1G
- Posts: 61
- Joined: Fri Apr 06, 2018 11:03 am
Re: Test 2 Question 4
To further explain , it is from n=x to n=2
You would use the
wav= h/m*v put this into terms of v and solve using 700nm given for wavelength
then when v is found find energy E=hv
after this En=-hR/n^2 , put in terms of n and solve
when you get n then use the En=-hR/n^2 equation again with that n found and the n=2 given to find the difference between the two.
-Jimmy Lira 1G
You would use the
wav= h/m*v put this into terms of v and solve using 700nm given for wavelength
then when v is found find energy E=hv
after this En=-hR/n^2 , put in terms of n and solve
when you get n then use the En=-hR/n^2 equation again with that n found and the n=2 given to find the difference between the two.
-Jimmy Lira 1G
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Test 2 Question 4
It's just asking for the difference in energy between these two states so you do not need to set up the Rydberg equation. The difference in energy comes from the photon with wavelength 400nm so you get the answer by solving E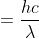 .
.
-
Sara Veerman-1H
- Posts: 31
- Joined: Fri Apr 06, 2018 11:05 am
-
Liliana Rosales 1E
- Posts: 36
- Joined: Wed Nov 15, 2017 3:03 am
Re: Test 2 Question 4
Sara Veerman-1H wrote:Lilianna, I believe the answer to this problem is 2.84x10^-19 J
Thank you Sara!
-
Salena Chowdri 1I
- Posts: 66
- Joined: Fri Apr 06, 2018 11:02 am
Re: Test 2 Question 4
Most students attempted to solve this problem using Rydberg's, however that is not necessary. The question already mentions that energy is released in the form of light at 400nm. The energy produced by this wavelength is the "energy difference". So all we need is to plug in the wave length into the equation: E= hv ==> E= (hc/wavelength)
-
Yitzchak Jacobson 1F
- Posts: 31
- Joined: Wed Nov 22, 2017 3:00 am
Re: Test 2 Question 4
Hello, At first I assumed that you would use the ryberg equation to solve the problem, but then I realized you can just solve it by using the equation
E=hc/lamda,
Really hopes this helps :)
E=hc/lamda,
Really hopes this helps :)
Return to “Properties of Light”
Who is online
Users browsing this forum: No registered users and 5 guests

