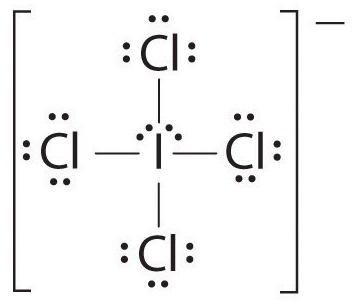
Postby Heung Ching Chia 1E » Sun May 27, 2018 11:38 pm
ICl4- has 36 electrons in total. You first form a skeletal structure (with iodine (least electronegative atom) as your central atom)and so there are 4 single bonds connecting the iodine to each chlorine and that means you used up 8 electrons (28 e- left). You then add 6 electrons to each chlorine atom to form a complete octet because chlorine is more electronegative than iodine, so its pull on the electrons will be stronger and therefore you add the remaining electrons to chlorine first. You are then left with 28-(6*4)=4 electrons. The 4 electrons then go onto the central atom iodine as lone pair electrons. Iodine is an element after the 3rd period, so it can have an expanded octet.
Lastly, you can check formal charge to see if this is the best lewis structure.