De Broglie Equation
Moderators: Chem_Mod, Chem_Admin
-
Caitlin_Murphy_3C
- Posts: 36
- Joined: Fri Sep 28, 2018 12:23 am
De Broglie Equation
In class on Friday, Dr. Lavelle introduced the De Broglie Equation to show the relationship between wavelength of any moving object with its mass and velocity. When doing the class examples, I noticed the mass of objects were kept in kilograms. Do all masses need to be in kilograms when using the De Broglie Equation?
-
armintaheri
- Posts: 68
- Joined: Fri Sep 28, 2018 12:26 am
Re: De Broglie Equation
The equation contains the Planck constant, which is usually given in 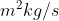 . So you need to plug in masses in kilograms, otherwise the units won't cancel out properly.
. So you need to plug in masses in kilograms, otherwise the units won't cancel out properly.
-
Kathryn 1F
- Posts: 66
- Joined: Fri Sep 28, 2018 12:19 am
Re: De Broglie Equation
Hello!
In equations when you use metric units, people use kilograms instead of grams, just because kg is the standard SI unit for mass, so its what equations are based on.
This is pretty conventional but its because most things we would weigh in the classical sense, like everyday objects, are better suited to being measured in kg.
In equations when you use metric units, people use kilograms instead of grams, just because kg is the standard SI unit for mass, so its what equations are based on.
This is pretty conventional but its because most things we would weigh in the classical sense, like everyday objects, are better suited to being measured in kg.
-
SydBenedict2H
- Posts: 60
- Joined: Wed Nov 08, 2017 3:00 am
Re: De Broglie Equation
Im slightly confused as to why Kg is the standard SI unit for mass when all of the mole conversions and problems weve been doing thus far have been in grams?
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: De Broglie Equation
Just make sure to be consistent in units. I think kilograms are used because Joules are a more physics-based unit, and physicists like to use kilograms. For example, Newtons are a derived unit in physics that also use kilograms (kg*m*s^-2). Physicists and chemists don't always work in the same magnitudes (e.g. g vs. kg, so just be aware!).
-
Luc Lorain 1L
- Posts: 59
- Joined: Fri Sep 28, 2018 12:18 am
Re: De Broglie Equation
Pretty much what is posted above. In addition, we've been working with grams quite a bit because molecular weights are typically given in the units grams per mole, allowing for quick stoichiometric conversions.
-
Claudia Luong 4K
- Posts: 59
- Joined: Fri Sep 28, 2018 12:25 am
Re: De Broglie Equation
Yes, as others have stated before, you should use kg in order to keep consistency since Planck's constant is in kg. I believe that during the lecture, Dr. Lavelle also said we will use kg due to kg being the standard SI unit for mass!
Return to “Properties of Light”
Who is online
Users browsing this forum: No registered users and 2 guests

