Does degeneracy = 0?
Moderators: Chem_Mod, Chem_Admin
-
Mara Lockhart 3J
- Posts: 53
- Joined: Fri Sep 26, 2014 2:02 pm
Does degeneracy = 0?
Will or does degeneracy, W, ever equal 0? If so, under what circumstances would make it 0 (a specific phase, isothermal, isobaric, reversible, irreversible)?
-
Neil DSilva 1L
- Posts: 70
- Joined: Fri Sep 26, 2014 2:02 pm
Re: Does degeneracy = 0?
I have no idea what the right answer to this would be, but I think the degeneracy is never going to be zero. If you consider a two-state system, the degeneracy is 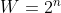 . No matter what you plug in for n, you're never going to get to zero. If you plugged in zero, you would get a degeneracy of 1. You can't have a "zero-state" system. Also, the natural log function, which we use to get entropy, is only defined for
. No matter what you plug in for n, you're never going to get to zero. If you plugged in zero, you would get a degeneracy of 1. You can't have a "zero-state" system. Also, the natural log function, which we use to get entropy, is only defined for 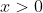 . So if
. So if 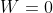 (hypothetically), entropy would be undefined.
(hypothetically), entropy would be undefined.
-
Justin Le 2I
- Posts: 142
- Joined: Fri Sep 26, 2014 2:02 pm
Re: Does degeneracy = 0?
Degeneracy can never equal 0. The lowest value it can get to is 1 which occurs at absolute zero because the system only has configuration without any kind of energy. Entropy, however, can equal 0 when w=1.
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Does degeneracy = 0?
The above posts are correct. Degeneracy can also be referred to as "microstates", describing the number of states of the system. If there is any tiny change in any variable, such as a molecule's energy, position, or orientation, then we get a different state.
It makes no sense for a system to have 0 states, since then it wouldn't exist at all. The minimum is 1 state, and when W=1, S=k*ln(W)=0 and there is no entropy (Third Law of Thermodynamics)
It makes no sense for a system to have 0 states, since then it wouldn't exist at all. The minimum is 1 state, and when W=1, S=k*ln(W)=0 and there is no entropy (Third Law of Thermodynamics)
-
Mara Lockhart 3J
- Posts: 53
- Joined: Fri Sep 26, 2014 2:02 pm
Re: Does degeneracy = 0?
Okay, thank you! Could degeneracy be undefined ever? Or is degeneracy always going to be a whole number greater than 0?
-
Neil DSilva 1L
- Posts: 70
- Joined: Fri Sep 26, 2014 2:02 pm
-
DylanTookey_1D
- Posts: 21
- Joined: Fri Sep 25, 2015 3:00 am
Re: Does degeneracy = 0?
I think degeneracy must be a whole number (you can't have 1/2 of a way to get to an energy level). For this reason, I believe that the lowest value that you can get for degeneracy is 1 because if it were equal to 0 then there would be no way to get to that energy state. Then if there was no way to get to an energy state, I would assume that it does not exist. I think (for this class at least), it's safe to assume that degeneracy will always be greater than or equal to 1.
Who is online
Users browsing this forum: No registered users and 1 guest

