Sigma bonds
Moderators: Chem_Mod, Chem_Admin
-
Marsenne Cabral 1A
- Posts: 59
- Joined: Fri Sep 28, 2018 12:19 am
-
Marcela Udave 1F
- Posts: 66
- Joined: Fri Sep 28, 2018 12:16 am
-
Shash Khemka 1K
- Posts: 38
- Joined: Fri Sep 28, 2018 12:18 am
Re: Sigma bonds
Sigma bonds can either have an S-S orbital bond, S-P orbital bond, or P-P orbital bond. They connect and overlap end-to-end. The S-S orbital bond looks like a Venn Diagram, the S-P orbital bond looks like a circle overlapping one portion of a dumbbell, and the P-P orbital bond looks like two dumbbells overlapping just by their tips.
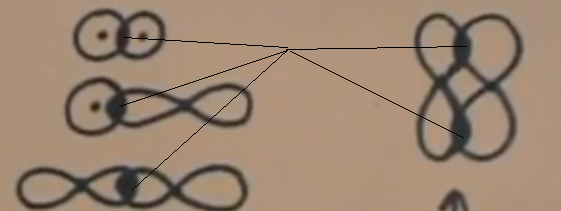
[Drawing on the right is of Pi Bond]

[Drawing on the right is of Pi Bond]
-
Anmol_cheema_2F
- Posts: 60
- Joined: Fri Sep 28, 2018 12:26 am
-
Elle_Mendelson_2K
- Posts: 72
- Joined: Fri Sep 28, 2018 12:28 am
Re: Sigma bonds
Sigma bonds are about the internuclear axis and overlap on top of one another (like two pancakes) whereas pi bonds interlap side to side like (two vertical rods)
-
sarahartzell1A
- Posts: 56
- Joined: Fri Sep 28, 2018 12:17 am
-
Emily Ng_4C
- Posts: 65
- Joined: Fri Sep 28, 2018 12:17 am
Re: Sigma bonds
Sigma bonds are when two orbitals bond at one point, while pi bonds are when two orbitals bond at two points.
Who is online
Users browsing this forum: No registered users and 3 guests

