For example, in the equation 4NH3(g) + 5O2(g)
Result of Only Adding One of the Reactants
Moderators: Chem_Mod, Chem_Admin
-
Joon Chang 2F
- Posts: 59
- Joined: Fri Sep 28, 2018 12:25 am
Result of Only Adding One of the Reactants
Can someone explain what will happen if only one of the reactants is added to a chemical equation?
For example, in the equation 4NH3(g) + 5O2(g)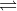 4NO(g) + 6H2O(g), if only NH3 is added to the equation, will equilibrium shift toward the products?
4NO(g) + 6H2O(g), if only NH3 is added to the equation, will equilibrium shift toward the products?
For example, in the equation 4NH3(g) + 5O2(g)
Re: Result of Only Adding One of the Reactants
Yes, because by increasing the concentration of any one reactant, the denominator (i.e. the product of reactant concentrations) of the quotient Q will become greater than the denominator of K at equilibrium. Thus, Q < K, and reaction will proceed to balance this larger denominator out by creating more product.
-
Timothy_Yueh_4L
- Posts: 57
- Joined: Fri Sep 28, 2018 12:28 am
Re: Result of Only Adding One of the Reactants
By adding more NH3 to the chemical reaction, you increase the concentration of the that particular chemical on the reactants side which gives an Q constant that is lower than the equilibrium constant, Kc. This will result with the forward reaction being favored as more product will be formed until the original equilibrium constant is attained.
-
Melody P 2B
- Posts: 35
- Joined: Fri Sep 29, 2017 7:06 am
Re: Result of Only Adding One of the Reactants
Then, if you were to remove some reactant would the reaction shift left? Towards the reactants to compensate for the loss?
-
Edward Xie 2E
- Posts: 63
- Joined: Fri Sep 28, 2018 12:23 am
Re: Result of Only Adding One of the Reactants
Then, if you were to remove some reactant would the reaction shift left? Towards the reactants to compensate for the loss?
Yep, since Q > K the reaction will shift towards the reactants.
Return to “Applying Le Chatelier's Principle to Changes in Chemical & Physical Conditions”
Who is online
Users browsing this forum: No registered users and 6 guests

