Kp vs Kc
Moderators: Chem_Mod, Chem_Admin
-
Katherine Grillo 1B
- Posts: 69
- Joined: Fri Sep 28, 2018 12:28 am
Kp vs Kc
When you're calculating K and all the R and P are gases, does it really matter whether or not you use partial pressure or molar concentrations? You would get the same calculation right?
-
Dhwani Krishnan 1G
- Posts: 63
- Joined: Fri Sep 28, 2018 12:17 am
Re: Kp vs Kc
If all the reactants/products are gases, it doesn't matter which you use. However, Kc and Kp are not always equal! This is due to concentration and Pressure being related via the Ideal Gas Law (PV = nRT)
This link explains the technicalities of it well: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Equilibria/Chemical_Equilibria/Calculating_An_Equilibrium_Concentrations/Writing_Equilibrium_Constant_Expressions_Involving_Gases/Gas_Equilibrium_Constants%3A_Kc_And_Kp
This link explains the technicalities of it well: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Equilibria/Chemical_Equilibria/Calculating_An_Equilibrium_Concentrations/Writing_Equilibrium_Constant_Expressions_Involving_Gases/Gas_Equilibrium_Constants%3A_Kc_And_Kp
-
Luc Lorain 1L
- Posts: 59
- Joined: Fri Sep 28, 2018 12:18 am
Re: Kp vs Kc
Like the poster above noted, the equilibrium constant for the concentration of species in gaseous equilibrium and their partial pressures may be the same value, but this often not the case. Partial pressure and concentration within a system are directly proportional in value, meaning that while proportionally species have the same PP and concentration, their actual values may be multiple magnitudes larger or smaller.
For instance, take the hypothetical gaseous reaction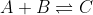 , at 200K in a 1.00 L reaction vessel. If there were 0.2 moles of A, 0.3 moles of B, and 0.5 moles of C at equilibrium, then 20% of the partial pressure in the system is due to A, 30% due to B, and 50% due to C. Likewise, the system is also 20% A, 30%B, and 50% C by concentration. However, this is not to state that concentration and PP are equal; using the ideal gas law will allow us translate concentration into PP and then find equilibrium concentration values.
, at 200K in a 1.00 L reaction vessel. If there were 0.2 moles of A, 0.3 moles of B, and 0.5 moles of C at equilibrium, then 20% of the partial pressure in the system is due to A, 30% due to B, and 50% due to C. Likewise, the system is also 20% A, 30%B, and 50% C by concentration. However, this is not to state that concentration and PP are equal; using the ideal gas law will allow us translate concentration into PP and then find equilibrium concentration values.
For instance, take the hypothetical gaseous reaction
-
Jason Ye 2I
- Posts: 33
- Joined: Fri Sep 28, 2018 12:22 am
-
Kessandra Ng 1K
- Posts: 67
- Joined: Fri Sep 28, 2018 12:25 am
Re: Kp vs Kc
To add to this, the question would usually give you either concentration values or partial pressure values and when given one or the other, you would know whether to use Kc or Kp.
-
Yiting_Gong_4L
- Posts: 69
- Joined: Fri Sep 28, 2018 12:25 am
-
Ashe Chen 2C
- Posts: 31
- Joined: Mon Jan 07, 2019 8:23 am
Return to “Equilibrium Constants & Calculating Concentrations”
Who is online
Users browsing this forum: No registered users and 16 guests

