U and its relations to work
Moderators: Chem_Mod, Chem_Admin
-
Julia Go 2L
- Posts: 60
- Joined: Sun Sep 30, 2018 12:17 am
- Been upvoted: 1 time
-
Cynthia Aragon 1B
- Posts: 47
- Joined: Mon Apr 09, 2018 1:38 pm
Re: U and its relations to work
U, stands for the internal energy of a system which can be altered by heat flow and work.
If heat flows into the system; work is done on the system, and its internal energy increases, ΔU > 0.
If heat flows out of the system, work is done by the system, and its internal energy decreases, ΔU < 0
Hope this helps!
If heat flows into the system; work is done on the system, and its internal energy increases, ΔU > 0.
If heat flows out of the system, work is done by the system, and its internal energy decreases, ΔU < 0
Hope this helps!
-
Annalyn Diaz 1J
- Posts: 61
- Joined: Fri Sep 28, 2018 12:15 am
Re: U and its relations to work
Additionally in a closed system, internal energy relates to work in this equation:
delta U = q + w
delta U = q + w
-
Danny Zhang 4L
- Posts: 62
- Joined: Fri Sep 28, 2018 12:26 am
Re: U and its relations to work
U represents the internal energy of a system. Its relation to work can be seen in the first law of thermodynamics: change in U = q(heat exchanged) + w(work done on or by the system).
-
Lopez_Melissa-Dis4E
- Posts: 66
- Joined: Fri Sep 28, 2018 12:20 am
Re: U and its relations to work
The equation that relates U to work is delta U= q+w, U represents the change in internal energy.
-
Sydney To 1D
- Posts: 60
- Joined: Fri Sep 28, 2018 12:15 am
Re: U and its relations to work
U is a state property and is the internal energy of a system. Changes in internal energy are a function of work. The energy of a closed system can be changed by heating/cooling or compression/expansion.  U=q + w. If
U=q + w. If  V is zero (constant volume), delta U=
V is zero (constant volume), delta U= 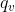 . If
. If  P=0 (constant pressure), then
P=0 (constant pressure), then  U=
U= H-P
H-P V.
V.
-
Jordan Lo 2A
- Posts: 85
- Joined: Fri Sep 28, 2018 12:25 am
-
Eruchi Okpara 2E
- Posts: 53
- Joined: Fri Sep 28, 2018 12:28 am
-
MadelynNguyen1F
- Posts: 59
- Joined: Fri Apr 06, 2018 11:04 am
Return to “Phase Changes & Related Calculations”
Who is online
Users browsing this forum: No registered users and 12 guests

