Calculating G if H and S are gven
Moderators: Chem_Mod, Chem_Admin
-
Kimberly 1H
- Posts: 63
- Joined: Fri Sep 28, 2018 12:17 am
- Been upvoted: 1 time
Calculating G if H and S are gven
Can someone please explain why we assume  G is 0 when H and S are given?
G is 0 when H and S are given?
-
whitney_2C
- Posts: 74
- Joined: Fri Sep 28, 2018 12:28 am
Re: Calculating G if H and S are gven
We aren't assuming that the Gibbs Free Energy is equal to 0, rather we are putting in 0 for its value to see when 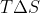 and
and  are equal to one another in order to determine at what temperatures the Gibbs Free Energy would be negative (spontaneous reaction) or positive (non spontaneous reaction).
are equal to one another in order to determine at what temperatures the Gibbs Free Energy would be negative (spontaneous reaction) or positive (non spontaneous reaction).
Re: Calculating G if H and S are gven
If we have the value when the system is in equilibrium and delta G =0, then we can find the behavior (in terms of spontaneity) for any temperatures above or below that value.
-
annabel 2A
- Posts: 67
- Joined: Fri Sep 28, 2018 12:18 am
Re: Calculating G if H and S are gven
Why can we use the same deltaH and deltaS to calculate deltaG at any temperature? (like in this problem: 9.53 Calculate the change in molar Gibbs free energy for the process NH3(l) --> NH3 (g) at 1atm and (a) 15.0 C; (b) 45. C (see Tables 8.3 and 9.1 for standard enthalpy and entropy of vaporization))
I thought that deltaH and deltaS would be different for different temperatures? Are the changes in enthalpy and entropy for a phase change the same at any temperature?
I thought that deltaH and deltaS would be different for different temperatures? Are the changes in enthalpy and entropy for a phase change the same at any temperature?
Return to “Gibbs Free Energy Concepts and Calculations”
Who is online
Users browsing this forum: No registered users and 5 guests

