Hello!
The question reads "Lines in the Balmer series of the hydrogen spectrum are observed at 656.3, 486.1, 434.0, and 410.2 nm. What is the wavelength of the next line in the series?"
^^^ I have tried using Professor Lavelle's method from his Course Reader where En = -hR/n , but cannot seem to obtain the correct answer. I tried it with the equation in the book and was able to achieve the right answer.
I was wondering whether Professor Lavelle's method can be applied to this problem or are we supposed to the use the equation that is presented to us in the book?
Thank you for the help!
Confused on Problem 1.57 (regarding the Balmer Series)
Moderators: Chem_Mod, Chem_Admin
-
Chem_Mod
- Posts: 23858
- Joined: Thu Aug 04, 2011 1:53 pm
- Has upvoted: 1253 times
Re: Confused on Problem 1.57 (regarding the Balmer Series)
Using the equation in the course reader should get you to the correct solution assuming you keep track of your initial and final states correctly and turn the energy loss of the electron into a positive energy for the radiation emitted. The next line in the series should come from the n = 7 transitioning to n = 2. The change in energy can then be expressed as =-\frac{hR}{4}+\frac{hR}{49})
That change in energy should be a negative value which represents the loss in energy of the electron. The energy of the emitted radiation will be that value as a positive. From there you can use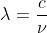 and
and 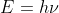 to find the correct wavelength.
to find the correct wavelength.
That change in energy should be a negative value which represents the loss in energy of the electron. The energy of the emitted radiation will be that value as a positive. From there you can use
-
nanditasundarapandian1D
- Posts: 21
- Joined: Sat Jul 22, 2017 3:01 am
Re: Confused on Problem 1.57 (regarding the Balmer Series)
Can you explain how to solve this problem in general?
-
Rachel N 1I
- Posts: 48
- Joined: Thu Jul 27, 2017 3:00 am
Re: Confused on Problem 1.57 (regarding the Balmer Series)
The series of the wavelengths given will help you find the final energy level. Since this is a Balmer series, you already know that n1 =2, and by counting the numbers, you can see that n2 =7. So using the equation E = -hR/n^2 you can find the energy of both n1=2 and n2=2. Finally, you can use Ef - Ei to get the change in energy. Once that energy is found plug it into the equation E = hc/wavelength.
-
Ashley Kim
- Posts: 62
- Joined: Fri Sep 28, 2018 12:19 am
- Been upvoted: 1 time
Re: Confused on Problem 1.57 (regarding the Balmer Series)
Hello, I'm reviving an old post. However, I don't understand why you can figure out that n2=7 using the Balmer series. Can someone please explain?
Re: Confused on Problem 1.57 (regarding the Balmer Series)
I believe it is because if you count the first given wavelength would be n = 3, then n=4 and so on... does that help?
-
Katie_Duong_1D
- Posts: 69
- Joined: Fri Sep 28, 2018 12:27 am
Re: Confused on Problem 1.57 (regarding the Balmer Series)
In a Balmer series, n1=2 and n2=7. We can find n2 by using the equation V = R(1/n1^2 - 1/n2^2). V = 3.29 x 10^29 Hz (Rydberg's constant) x (1/4 - 1/39) = 7.55 x 10^14 Hz.
Then we can plug v into c = lambda x v and solve for lambda to find wavelength.
lambda = c/v = (3.00 x 10^8 m/s)/(7.55 x 10^14 s^-1) = 3.97 x 10^-7 m
Wavelength is generally written in nanometers, nm = 10^-9 m, so the wavelength of the next line is 397 nm.
Then we can plug v into c = lambda x v and solve for lambda to find wavelength.
lambda = c/v = (3.00 x 10^8 m/s)/(7.55 x 10^14 s^-1) = 3.97 x 10^-7 m
Wavelength is generally written in nanometers, nm = 10^-9 m, so the wavelength of the next line is 397 nm.
-
Nada AbouHaiba 1I
- Posts: 77
- Joined: Fri Sep 28, 2018 12:28 am
- Been upvoted: 1 time
Re: Confused on Problem 1.57 (regarding the Balmer Series)
Ashley Kim wrote:Hello, I'm reviving an old post. However, I don't understand why you can figure out that n2=7 using the Balmer series. Can someone please explain?
I'm also confused. I thought the balmer series started at 2, so wouldn't it be:
656.3 (n2), 486.1 (n3), 434.0 (n4), 410.2 (n5)...
so wouldn't the next one be n6 not n7?
Sorry this may be a stupid question but I'm not sure what I'm missing
thank you in advance!
-
Nada AbouHaiba 1I
- Posts: 77
- Joined: Fri Sep 28, 2018 12:28 am
- Been upvoted: 1 time
Re: Confused on Problem 1.57 (regarding the Balmer Series)
okay so I dug around a little and found a post that does a good job of explaining it, I'll link it below just in case anyone else is still confused :)
viewtopic.php?t=31402
viewtopic.php?t=31402
-
brennayoung
- Posts: 51
- Joined: Fri Sep 20, 2019 12:17 am
Re: Confused on Problem 1.57 (regarding the Balmer Series)
How can you tell that it is n=7 based off of the numbers? I found a couple diagrams online that show 410.2 as n=6.
Return to “Bohr Frequency Condition, H-Atom , Atomic Spectroscopy”
Who is online
Users browsing this forum: No registered users and 1 guest

