Rate of Formation vs Unique Rate
Moderators: Chem_Mod, Chem_Admin
-
beckyolmedo1G
- Posts: 34
- Joined: Fri Sep 28, 2018 12:21 am
-
Chloe Qiao 4C
- Posts: 65
- Joined: Fri Sep 28, 2018 12:27 am
Re: Rate of Formation vs Unique Rate
I believe they are not the same. Rate of formation depends on the coefficient of the products, but unique rate does not.
-
Pegah Nasseri 1K
- Posts: 100
- Joined: Wed Feb 27, 2019 12:15 am
Re: Rate of Formation vs Unique Rate
Given the reaction 2A + B -> 3C, the rate following the rate law would be 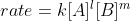 . Using the unique rate instead of the rate law, you would calculate the rate by plugging in the values for any of these:
. Using the unique rate instead of the rate law, you would calculate the rate by plugging in the values for any of these: 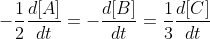 . The unique rate can be calculated using the rate of formation of A or B, or by calculating the rate of decomposition of C (which would be the negative value of the other two rate of formations). The unique rate of formation takes into account the stoichiometric coefficients of the reaction while the rate law does NOT.
. The unique rate can be calculated using the rate of formation of A or B, or by calculating the rate of decomposition of C (which would be the negative value of the other two rate of formations). The unique rate of formation takes into account the stoichiometric coefficients of the reaction while the rate law does NOT.
-
Morgan Carrington 2H
- Posts: 54
- Joined: Wed Nov 14, 2018 12:22 am
Re: Rate of Formation vs Unique Rate
Chloe Qiao 4C wrote:I believe they are not the same. Rate of formation depends on the coefficient of the products, but unique rate does not.
How would you determine the unique rate law if these two values are not the same? I'm super lost by how to differentiate between these two values.
Who is online
Users browsing this forum: No registered users and 7 guests

