calculating standard cell potential
Moderators: Chem_Mod, Chem_Admin
-
hannahdaijo_4H
- Posts: 48
- Joined: Fri Sep 28, 2018 12:27 am
calculating standard cell potential
Why is standard cell potential sometimes equal to the difference between the standard potentials of the two electrodes and sometimes its the sum of the standard potentials of the cathode and the anodes?
-
AnnaYan_1l
- Posts: 96
- Joined: Fri Apr 06, 2018 11:05 am
- Been upvoted: 1 time
Re: calculating standard cell potential
Half reactions are usually given as reduction reactions.
It's the difference when you're keeping both anode and cathode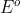 's at their reduction potentials
's at their reduction potentials
It's added when you flip the anode reaction (making it go from a reduction to oxidation) and thus flip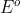 .
.
Hope that helps!
It's the difference when you're keeping both anode and cathode
It's added when you flip the anode reaction (making it go from a reduction to oxidation) and thus flip
Hope that helps!
-
Kassidy Tran 1E
- Posts: 77
- Joined: Fri Sep 28, 2018 12:15 am
Re: calculating standard cell potential
There are two ways of calculating standard potential (E). You can either use E_cell = E_cathode - E_anode, in which you just take the standard reduction potentials from the chart and plug them into the equation as is, without changing the signs, or you can add up the half reactions and their standard potentials. In the second method, you would have to flip the anode so that the electrons cancel out, and because you flipped the reaction, you need to flip the sign of the standard reduction potential. In this case you would just add the two E values together as you do with the half reaction equations.
Re: calculating standard cell potential
YES this finally made sense to me now thank thank you so much! i could never tell when to flip or not thank you!
-
SydBenedict2H
- Posts: 60
- Joined: Wed Nov 08, 2017 3:00 am
Re: calculating standard cell potential
Just remember that if you use the overall net equation for E you leave the signs and if you're adding up form had reactions etc. you have already flipped the sign so just add.
Re: calculating standard cell potential
If you subtract cathode - anode, you don't flip the signs. If you add them, then you would have to flip the sign of the anode.
Return to “Balancing Redox Reactions”
Who is online
Users browsing this forum: No registered users and 17 guests

