Jasmine Reddy DIS 1E wrote:When taking values from the periodic table (ex. for calculating molar mass), how many significant figures do you round to?
Hi Jasmine,
Lina said that Lina takes all of the molar mass digits the periodic table has to offer when solving problems. Working with all the molar mass digits and then rounding/applying significant figures at the end of the problem ensures the accuracy of the final answer.
However, personally, when reading molar masses, I like to take only 2-3 digits after the decimal. While not as accurate as the previous method of taking the entire molar mass, rounding molar masses to the 2nd or 3rd decimal makes memorizing common molar masses much easier. After you do enough problems, you probably won't need to refer to the periodic tables for Hydrogen (1.01g/mol), Oxygen (16g/mol), Carbon (12.01g/mol), Nitrogen (14.01 g/mol), and whatever other molar masses you can hold in your brain. Which method you use is up for personal preference: do you want to be a little more accurate? or a little more efficient?
Keep in mind though: Though I take 1.01g/mol for Hydrogen's molar mass, I treat that value as an exact value. That means 1.01g/mol has infinite significant figures. If a question asked me to find the mass of 2.000 mol of H
2 I would include 4 sig figs in my final answer:
*(\frac{1.01g}{1molH})=4.040g)
2.000 has 4 sig figs
2
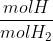
has infinity sig figs
1.01g/mol also has infinity sig figs
And so my final answer would come out with the same sig figs as the term with the least sig figs, which is 2.000molH
2 with 4 sig figs.

