De Broglie's Equation
Moderators: Chem_Mod, Chem_Admin
-
Luyan Zhang - 2D
- Posts: 103
- Joined: Sat Jul 20, 2019 12:16 am
De Broglie's Equation
If the wavelength of something with a large mass cannot be detected by lab equipments, then how did de Broglie come up with this relation? Or is it all theoretical?
-
Camellia Liu 1J
- Posts: 51
- Joined: Sat Aug 24, 2019 12:15 am
Re: De Broglie's Equation
When De Broglie derived his equation, he didn't actually have any experimental data. Instead, he based it all on well-established theories and equations like Einstein's E=mc^2 and Planck's equation, E=hv. I don't think we usually use De Broglie's equation to apply to objects with very large masses. We usually use it to investigate/apply to particles, atoms and molecules.
-
Milisuryani Santoso 1L
- Posts: 44
- Joined: Thu Jul 11, 2019 12:15 am
Re: De Broglie's Equation
Well, De Broglie's Equation is accurate when it comes to measuring small things like electrons, neutrons, and protons, so we'd assume it'd work for anything that has rest mass. However, as technology is as it is now, we don't have the tools that can measure wavelengths as small as the ones objects with large masses would theoretically emit. Thus, we can't really prove or disprove if it 'works' for things with large masses.
-
Sidharth D 1E
- Posts: 98
- Joined: Sat Aug 24, 2019 12:17 am
Re: De Broglie's Equation
For example, for the problem we did with the 0.1 kg baseball moving at 35 m/s, the resulting wavelength that this mass would emit is 1.9 x 10-34 m, which is a wavelength we cannot measure and, therefore, prove or disprove.
-
Jordan Ziegler 2J
- Posts: 59
- Joined: Sat Aug 17, 2019 12:15 am
Re: De Broglie's Equation
Can anyone explain or outline the physics-based proof for the DeBroglie Equation?
I understand that it shouldn't be used for light and that it applies to electrons (thus you shouldn't use it in relation to equations like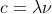 or E=h
or E=h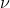 ) but I'm interested in learning more!
) but I'm interested in learning more!
I understand that it shouldn't be used for light and that it applies to electrons (thus you shouldn't use it in relation to equations like
Return to “DeBroglie Equation”
Who is online
Users browsing this forum: No registered users and 8 guests

