Combustion
Moderators: Chem_Mod, Chem_Admin
-
Lelija Kazlauskas 3J
- Posts: 50
- Joined: Thu Jul 11, 2019 12:16 am
Combustion
What exactly is combustion, and how are we supposed to represent it in a chemical equation?
-
Kyla Grunden 1L
- Posts: 46
- Joined: Sat Sep 07, 2019 12:16 am
Re: Combustion
A combustion reaction occurs when a compound is reacted with oxygen, releasing heat and light. I don't think there's any way, or reason, to represent that a reaction is combustion, but the second reactant is always oxygen gas.
Hope this helps!
Hope this helps!
-
Kurtis Liang 3I
- Posts: 50
- Joined: Sat Aug 24, 2019 12:17 am
Re: Combustion
Combustion is the reaction between a hydrocarbon and oxygen to produce a CO2 and H2O.
-
Indy Bui 1l
- Posts: 99
- Joined: Sat Sep 07, 2019 12:19 am
Re: Combustion
Combustion is essentially the reaction between a hydrocarbon or organic compound (C3H4 for example) and oxygen gas (O2), this usually occurs through burning. The products that are formed are always Water (H2O) and Carbon Dioxide (CO2).
Here is a simple example combustion reaction with methane:
Unbalanced: CH4 + O2 --> CO2 + H2O
Balanced: CH4 + 2O2 --> CO2 + 2H2O
This is a really basic example but all you really need to know is that Oxygen is consumed and Carbon Dioxide and Water are always products.
Here is a simple example combustion reaction with methane:
Unbalanced: CH4 + O2 --> CO2 + H2O
Balanced: CH4 + 2O2 --> CO2 + 2H2O
This is a really basic example but all you really need to know is that Oxygen is consumed and Carbon Dioxide and Water are always products.
-
britthanul234
- Posts: 51
- Joined: Wed Sep 18, 2019 12:21 am
Re: Combustion
Combustion is simply a compound reacting with O2, creating the products, H20 and CO2. Combustion occurs through burning. Usually the question will specify whether the reaction is a combustion or not.
-
Robert Tran 1B
- Posts: 118
- Joined: Thu Jul 11, 2019 12:15 am
Re: Combustion
Combustion is when a compound reacts with oxygen to create water and carbon dioxide. In a chemical equation, the oxygen should be on the reactants side and the water and carbon dioxide should be on the products side.
Re: Combustion
Combustion is another word for burning. In a combustion reaction, the fuel (usually a hydrocarbon or other related molecule), burns with O2 gas. Combustion simply means reacting with O2 and could yield products such as water and carbon dioxide.
-
Maya Beal Dis 1D
- Posts: 100
- Joined: Sat Aug 17, 2019 12:16 am
- Been upvoted: 2 times
Re: Combustion
If in a problem it says it is a combustion reaction, that is how you know the products will be water and carbon dioxide. Often in combustion reactions the question will not give that information directly.
-
Subashni Rajiv 1K
- Posts: 101
- Joined: Wed Sep 18, 2019 12:16 am
Re: Combustion
The most important thing to know for a combustion reaction is that O2(gas) is always a reactant and the products are always H2O and CO2.
Re: Combustion
Combustion is when a carbon based compound reacts with oxygen to form carbon dioxide and water. To represent it in an equation you just have to react the initial compound with oxygen.
-
Tiffany Vo 3G
- Posts: 52
- Joined: Sat Aug 17, 2019 12:17 am
Re: Combustion
Combustion is an oxidation reaction, meaning it would involve oxygen because it receives an electron from the oxygen molecule. With most combustion reactions, they tend to form water and carbon dioxide.
-
Abigail Sanders 1E
- Posts: 112
- Joined: Wed Sep 11, 2019 12:16 am
Re: Combustion
Combustion is is a reaction with one molecule and oxygen that produces carbon dioxide and water. Combustion can also be referred to as the burning or oxidation of the organic molecule, or as oxidative metabolism.
-
BCaballero_4F
- Posts: 94
- Joined: Wed Nov 14, 2018 12:22 am
Re: Combustion
Combustion consists of burning, usually O^2 reacts with something else and CO^2 and H^2O are products.
-
Cynthia Rodas 4H
- Posts: 51
- Joined: Wed Sep 18, 2019 12:21 am
Re: Combustion
Combustion refers to a chemical reaction that includes burning in air. The most basic set-up is usually:
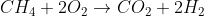 O
O
The main point is that the products will always include CO2 and H2O. It's just a matter of balancing the equation based on what is given to you.
The main point is that the products will always include CO2 and H2O. It's just a matter of balancing the equation based on what is given to you.
-
ABombino_2J
- Posts: 102
- Joined: Thu Jul 11, 2019 12:15 am
Re: Combustion
Combustion, or burning, occurs whenever a hydrocarbon interacts with Oxygen. The products are Carbon dioxide and Water. The more oxygen present the more successful the combustion will be.
-
ramiro_romero
- Posts: 90
- Joined: Sat Sep 07, 2019 12:16 am
Re: Combustion
A complete combustion will always be in the following format. (Fuel) + O2 -> CO2 + H2O.
-
pJimenez3F
- Posts: 45
- Joined: Wed Sep 18, 2019 12:18 am
Re: Combustion
if you see the word combustion you should automatically know that element is reacting with oxygen gas O2 to produce carbon dioxide CO2 and water H2O
-
HanaAwad_4B
- Posts: 45
- Joined: Fri Aug 30, 2019 12:18 am
Return to “Balancing Chemical Reactions”
Who is online
Users browsing this forum: No registered users and 3 guests

