HW 1B. 7
Moderators: Chem_Mod, Chem_Admin
HW 1B. 7
Sodium vapor lamps, used for public lighting, emit yellow light of wavelength 589 nm. How much energy is emitted by an excited sodium atom when it generates a photon? I looked in the solutions manual and it showed the formula E=h*frequency and c=wavelength*frequency, and then derived the formula E=hc*wavelength^-1. Can someone explain why or how this new formula was derived from the former two formulas? Thank you!
-
Shannon Asay 1C
- Posts: 102
- Joined: Fri Aug 09, 2019 12:16 am
Re: HW 1B. 7
So the two equations are E=hv and c=(wavelength)(v). If we solve for frequency in the speed of light equation, we get v=c/wavelength. You can plug that in for the frequency of the energy equation to get E=(h*c)/(wavelength)
Re: HW 1B. 7
First use the E=hc/wavelength formula to find the energy. Then, multiply that my the mols of Na and avagadros number to get the energy for 5g of Na. After that, multiple avagadros number to the number you calculated in part a to find the energy for 1mol of Na.
-
KBELTRAMI_1E
- Posts: 108
- Joined: Sat Jul 20, 2019 12:17 am
Re: HW 1B. 7
805307623 wrote:First use the E=hc/wavelength formula to find the energy. Then, multiply that my the mols of Na and avagadros number to get the energy for 5g of Na. After that, multiple avagadros number to the number you calculated in part a to find the energy for 1mol of Na.
So avogadro's number is how you get it in the correct mole qty?
-
alicechien_4F
- Posts: 104
- Joined: Sat Jul 20, 2019 12:15 am
Re: HW 1B. 7
KBELTRAMI_4I wrote:So avogadro's number is how you get it in the correct mole qty?
In part A, you're solving for the amount of energy emitted by one sodium atom. In part B, you're solving for the amount of energy emitted by 5.00 mg of sodium, so you have to convert 5.00 mg to moles, and then multiply by avogadro's number to see how many sodium atoms are in 5.00 mg. You're using avogadro's number to find the amount of sodium atoms so that you can multiply the number of atoms by the energy emitted per sodium atom from part A.
-
ValerieChavarin 4F
- Posts: 99
- Joined: Wed Sep 18, 2019 12:18 am
Re: HW 1B. 7
The new formula is derived from solving for the frequency in 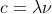 which gives you
which gives you 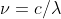 . You can then plug in this new value of
. You can then plug in this new value of 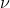 into E=h
into E=h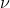 to get
to get 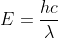
Re: HW 1B. 7
In the equation for part a derived in the solution manual, I understand the derivation and application, but where does the ^-1 come from? (the equation given is E=hcλ^-1)
Who is online
Users browsing this forum: No registered users and 1 guest

