1B #9
Moderators: Chem_Mod, Chem_Admin
-
Kelly Cai 4D
- Posts: 55
- Joined: Sat Jul 20, 2019 12:17 am
1B #9
A lamp rated at 32 W (1 W= 1 J/s) emits violet light of wavelength 420 nm. How many photons of violet light can the lamp generate in 2.0 s? How many moles of photons are emitted in that time interval?
-
SnehinRajkumar1L
- Posts: 101
- Joined: Thu Jul 11, 2019 12:15 am
Re: 1B #9
Calculate the frequency first using the wavelength of the light. Then, use that frequency to calculate the energy of an individual photon. Divide 32 by that value and multiply by 2 to get the number of photons emitted in 2 seconds. For the second part, divide by Avogadro's number to get number of moles.
-
Ashley Tran 2I
- Posts: 108
- Joined: Thu Jul 11, 2019 12:17 am
Re: 1B #9
Find energy per photon using the equation E=hc/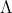
Multiply by 64 because there are 32 Watts per second and the prompt asks for 2 seconds (1 W = 1 J/s)
You will get 1.4 x 10^20 photons, then convert to moles using Avogadro's number.
The final answer will be 2.3 x 10^-4 mol photons
Multiply by 64 because there are 32 Watts per second and the prompt asks for 2 seconds (1 W = 1 J/s)
You will get 1.4 x 10^20 photons, then convert to moles using Avogadro's number.
The final answer will be 2.3 x 10^-4 mol photons
-
Ellis Song 4I
- Posts: 102
- Joined: Thu Jul 11, 2019 12:17 am
Re: 1B #9
32 W = 32 J/s in 2 sec -> 64 J
Use the equation E = hc/(wavelength) to find the energy per photon. You should get 4.73 x 10^-19 J/photon
Divide 64 J by that number to get the number of photons -- 1.35 x 10^20 photons
To find how many moles that is you just divide by Avogadro's number and should get 2.3 x 10^-4 mol photon
Use the equation E = hc/(wavelength) to find the energy per photon. You should get 4.73 x 10^-19 J/photon
Divide 64 J by that number to get the number of photons -- 1.35 x 10^20 photons
To find how many moles that is you just divide by Avogadro's number and should get 2.3 x 10^-4 mol photon
-
Anthony Hatashita 4H
- Posts: 103
- Joined: Wed Sep 18, 2019 12:21 am
Re: 1B #9
64 total Joules are emitted over 2 seconds, and we have all the information we need we need to find E=ch/(wavelength). After you find E, you can divide 64 by E to find out how many photons there are, and then you can divide that number by Avogadro's number, 6.022x10^23 to find the moles of photons.
Re: 1B #9
Simply just use the E=hc/(wavelength) formula to get the energy per photon and multiply that by (32*2), because 32W is given as the amount emitted in 1 second but its asking for the amount emitted in 2 seconds. Then, once you have the number of photons, divide by avagadros number to convert to moles of photons.
Return to “Properties of Light”
Who is online
Users browsing this forum: No registered users and 8 guests

