Question 1A.15
Moderators: Chem_Mod, Chem_Admin
-
Kimberly Koo 2I
- Posts: 99
- Joined: Sat Aug 17, 2019 12:17 am
Question 1A.15
Hi, could someone explain how to do question 1A.15 to me? The question says, in the ultraviolet spectrum of atomic hydrogen, a line is observed at 102.6 nm. Determine the values of n for the initial and final energy levels of the electron during the emission of energy that leads to this spectral line. Thanks so much!
Re: Question 1A.15
I had a hard time with this question too, I think we're supposed to use the Rydberg equation, but when I looked at the solutions guide I didn't fully understand it either, so other than that I don't know much, sorry!
-
Malia Shitabata 1F
- Posts: 127
- Joined: Sat Aug 17, 2019 12:17 am
Re: Question 1A.15
First you use 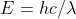 to calculate the change in energy then you use E=-hR/n^2 to calculate the energy at n=1. Plug those in to the energy equation where change in Energy=final energy-initial energy. Plug the final energy back into Rydberg's equation to solve for n as the final energy level.
to calculate the change in energy then you use E=-hR/n^2 to calculate the energy at n=1. Plug those in to the energy equation where change in Energy=final energy-initial energy. Plug the final energy back into Rydberg's equation to solve for n as the final energy level.
-
Kendall 3H
- Posts: 52
- Joined: Wed Sep 18, 2019 12:18 am
Re: Question 1A.15
First, you must recognize that the ultraviolet spectrum of atomic hydrogen is the lyman series and its n1 = 1. Then you use the equation v=c/lambda to calculate v. (v= 2.998 *10^8/ 102.6 * 10^8) Now, we can use the Rydberg equation to solve for n2. After plugging in all the numbers, you will get n2=3.
-
Cynthia Rodas 4H
- Posts: 51
- Joined: Wed Sep 18, 2019 12:21 am
Re: Question 1A.15
The equation we will be using to solve this problem is:
)
So the problem states that the spectral line 102.6 nm is in the ultraviolet spectrum of atomic hydrogen. We already know that the Lyman series is the set of lines in the ultraviolet region. Therefore, we know that n1 = 1. Next, we have to convert = 102.6 nm to meters. So,
= 102.6 nm to meters. So,
 = 102.6 nm x 10-9 m.
= 102.6 nm x 10-9 m.
We can find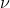 by using the equation
by using the equation 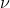 = c/
= c/
By doing so, we get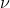 = 2.922 x 1015 s-1.
= 2.922 x 1015 s-1.
However, we are trying to find what n2 is since we already have n1, so we will manipulate the equation to get:
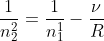
From there you just plug in everything and you'll get:
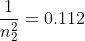
Keep in mind, we are trying to find what n2 is, so you manipulate the equation to get:
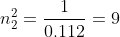
You find the square root to get n2 = 3.
So the values of n for the initial and final energy levels are 1 and 3 respectively.
So the problem states that the spectral line 102.6 nm is in the ultraviolet spectrum of atomic hydrogen. We already know that the Lyman series is the set of lines in the ultraviolet region. Therefore, we know that n1 = 1. Next, we have to convert
We can find
By doing so, we get
However, we are trying to find what n2 is since we already have n1, so we will manipulate the equation to get:
From there you just plug in everything and you'll get:
Keep in mind, we are trying to find what n2 is, so you manipulate the equation to get:
You find the square root to get n2 = 3.
So the values of n for the initial and final energy levels are 1 and 3 respectively.
-
Kendall 3H
- Posts: 52
- Joined: Wed Sep 18, 2019 12:18 am
Re: Question 1A.15
First, you must recognize that the ultraviolet spectrum of atomic hydrogen is the lyman series and its n1 = 1. Then you use the equation v=c/lambda to calculate v. (v= 2.998 *10^8/ 102.6 * 10^8) Now, we can use the Rydberg equation to solve for n2. After plugging in all the numbers, you will get n2=3.
Return to “Properties of Light”
Who is online
Users browsing this forum: No registered users and 9 guests

